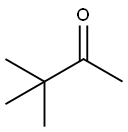
Chemical Properties
3,3-Dimethyl-2-butanone is a
colorless liquid with a light peppermint- or camphor-like odor. 3,3-Dimethyl-2-butanone is stable toward autoxidation; under standard conditions it does not
form hydroperoxides.
Only the a-methyl group can undergo condensation reactions. At the carbonyl group the
usual reactions (hydrogenation, reductive amination) can take place.
Uses
Pinacolone is a precursor to triazolylpinacolone, triadimefon, paclobutrazol, uniconazole and metribuzin. Furthermore, it acts as an intermediate for biologically active products such as antibacterial, antifungal, antiviral and antituberculous products. It is an important ketone in organic chemistry and participates in condensation, hydrogenation and reductive amination reactions.
Preparation
Pinacolone is prepared by distillation of Pinacolhydrate with diluted Sulfuric acid.
Synthesis Reference(s)
Journal of the American Chemical Society, 88, p. 5656, 1966
DOI: 10.1021/ja00975a058Organic Syntheses, Coll. Vol. 1, p. 462, 1941
Tetrahedron Letters, 30, p. 945, 1989
Synthesis
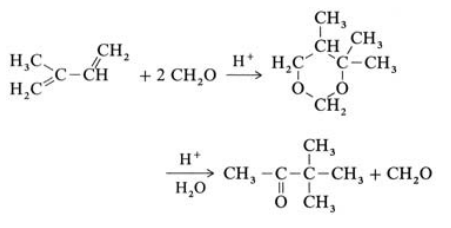
3,3-Dimethyl-2-butanone can be produced by the following routes: Hydrolysis of 4,4,5-trimethyl-1,3-dioxane, the product of the Prins reaction of isoprene with formaldehyde.
Purification Methods
Reflux the ketone with a little KMnO4. Dry it with CaSO4 and distil it. [Beilstein 1 IV 3310.]