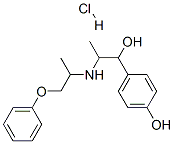
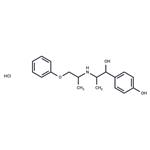
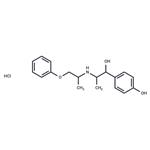
Description
Isoxsuprine is an adrenergic receptor modulator that has α-adrenergic receptor (α-AR) antagonist and β-AR agonist properties. It induces vasodilation of isolated equine common digital artery strips precontracted with norepinephrine, indicating an α-AR effect, and induces relaxation of isolated fowl cecum, an effect that can be blocked by the β-AR antagonist propranolol (Item Nos.
23349 |
17291). Isoxsuprine has antinociceptive effects in an acetic acid writhing test in mice. It also inhibits oxytocin-induced contractions in isolated rat uterus (IC
50 = 9.15 μM). It delays labor onset in rats by 31.63 hours when administered at a dose of 10 mg/kg per day on days 13 to 21 of gestation but increases heart rate with increasing concentration.
Chemical Properties
White Solid
Originator
Duvadilan,Duphar,France,1958
Uses
It is prescribed for the symptomatic relief of cerebrovascular insufficiency and improvement of circulation
Uses
As a vasilodator drug, Isoxsuprine Hydrochloride is used in veterinary medicine for the treatment of navicular syndrome and laminitis in horses. Isoxsuprine Hydrochloride has been shown to be a β-adrenoreceptor antagonist with β-adrenoreceptor agonistic properties, with both characteristics contributing to vasodilation and uterine relaxation. Studies suggest that Isoxsuprine Hydrochloride may be useful as a as a tocolytic agent in the treatment of preterm labour.
Definition
ChEBI: Isoxsuprine hydrochloride is an alkylbenzene.
Manufacturing Process
To a solution of 30.7 g (0.203 mol) of 1-phenoxy-2-aminopropane in 150 ml
of ethanol there was added 31.9 g (0.100 mol) of 1-(4'-benzyloxyphenyl)-2-
bromopropanone-1. The mixture was heated to boiling temperature and the
solution was then refluxed in a reflux condenser for 3 hours. Most of the
ethanol was then distilled off in vacuo, Then to the residue there was addedabout 150 ml of diethyl ether. The hydrogen bromide salt of 1-phenoxy-2-
aminopropane was filtered off and washed with diethyl ether.
The collected ethereal filtrates were acidified with 50 ml of 4 N hydrochloric
acid and this solution was stirred vigorously. The hydrochloride of 1-(4'-
benzyloxyphenyl)-2-(1'-methyl-2-phenoxy-ethylamino)propanone-1
precipitated out, was filtered off, washed with water and then with diethyl
ether. Then this substance was dried in vacuo. The yield was 37.7 g, i.e., 89%
of the theoretically possible yield, calculated on 1-(4'-benzyloxyphenyl)-2-
bromine propanone-1. This substance had a light yellow color and melted at
197 to 198°C, while decomposing.
Then 21.89 g of the hydrochloride salt was dissolved in 600 ml of 80%
aqueous ethanol. With the addition of a palladium carbon catalyst, this
solution was hydrogenated at room temperature under a hydrogen pressure of
about 1.1 atmospheres. After 2 mols hydrogen had been absorbed, the
catalyst was filtered off and the filtrate was evaporated in vacuo until
crystallization occurred. Then the crystals were dissolved by heating in the
smallest possible quantity of water and after cooling, the crystallized
substance was filtered off, washed with water and dried in vacuo. The yield
was 6.80 g, i.e., 39% of the theoretically possible yield. The resultant product
recrystallized from water melted at 203° to 204°C.
brand name
Vasodilan (Apothecon).
Therapeutic Function
Vasodilator
Veterinary Drugs and Treatments
Isoxsuprine is used in veterinary medicine principally for the treatment
of navicular
disease in horses; however, recent studies have
shown disappointing efficacy when used orally. It has been used in
humans for the treatment of cerebral vascular insufficiency, dysmenorrhea,
and premature labor, but efficacies are unproven for
these indications.
There has been an anecdotal report of isoxsuprine being helpful
for treating dogs with a Raynaud’s-like syndrome (periodic digital
cyanosis, onychogryphosis) (Carlotti 2002).
in vivo
animal stroke model, 1 mg/kg isoxsuprine was administered after a 90-minute occlusion of the right middle cerebral artery by iv injection at reperfusion. total infarct volume in isoxsuprine-treated group was 137±18 mm3 compared to 279±25 mm3 in control group [2].
References
1. r. s. ekert and c. g. macallister. isoxsuprine hydrochloride in the horse: a review. j. vet. pharmacol. ther. 2002, 25(2), 81-87 2. hill jw, thompson jf, carter mb. identification of isoxsuprine hydrochloride as a neuroprotectant in ischemic stroke through cell-based high-throughput screening. plos one. 2014 may 7; 9(5):e96761