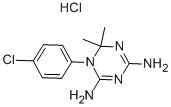
Description
Cycloguanil is the active metabolite of the antimalarial prodrug proguanil. Cycloguanil is formed from proguanil by the cytochrome P450 (CYP) isoforms CYP2C19 and CYP3A in human liver microsomes. It is an inhibitor of dihydrofolate reductase (DHFR; K
is = 1.5 and 0.79 nM for the
P. falciparum and
P. berghei enzymes, respectively). It is active against ten
P. falciparum field isolates (IC
50s = 0.12-1,400 μg/ml). Cycloguanil reduces parasitemia in a mouse model of
P. berghei infection (ED
50 = 2 mg/kg). It also reduces parasitemia in a rhesus monkey model of
P. cynomolgi infection when administered at a dose of 0.3 mg/kg.
Chemical Properties
Crystalline Solid
Uses
Labelled antimalarial drug
Definition
ChEBI: Cycloguanil hydrochloride is the hydrochloride salt of cycloguanil. It is an organic molecular entity and a hydrochloride. It contains a cycloguanil.
References
[1] foote s j, galatis d, cowman a f. amino acids in the dihydrofolate reductase-thymidylate synthase gene of plasmodium falciparum involved in cycloguanil resistance differ from those involved in pyrimethamine resistance[j]. proceedings of the national academy of sciences, 1990, 87(8): 3014-3017.
[2] blakley r l. dihydrofolate reductase[j]. encyclopedia of molecular medicine, 1984.
[3] birkett d j, rees d, andersson t, et al. in vitro proguanil activation to cycloguanil by human liver microsomes is mediated by cyp3a isoforms as well as by s‐mephenytoin hydroxylase[j]. british journal of clinical pharmacology, 1994, 37(5): 413-420.