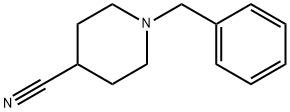
Synthesis

Step 1 Preparation of intermediate 1-benzyl-4-carbonylamide piperidine
To piperidine-4-carboxamide (16.5 g, 0.13 mol ) and K2CO3 (35.6 g, 0.26 mol) in EtOH (350 ml) was added benzyl bromide (22.0 g, 0.13 mol) And heated to reflux for 3 h, cooled to room temperature and filtered. The filtrate was evaporated in vacuo and H2O (200ml) was added. The aqueous layer was extracted with CH2Cl2 (3×150ml), the organic layers were combined, and Na2SO4Dry and filter. The solvent was evaporated in vacuo to give the product as a white solid (20.0 g, 71.0%).
Step 2 Preparation of intermediate 1-benzyl-4-cyanopiperidine
1-benzyl-4-carbonylamide piperidine (20.0 g, 91.7 mmol) Mixed with P2O5 (16.92, 119.2 mmol) and heated under argon at 180-200°C for 3 h, cooled to room temperature and added H2O (150 ml). The aqueous solution was basified by the careful addition of K2CO3 and then extracted with EtOAc (3× 150 ml). The organic extract was dried over Na2SO4, filtered and the solvent evaporated in vacuo to give a yellow oil (16.7 g, 90.9%).