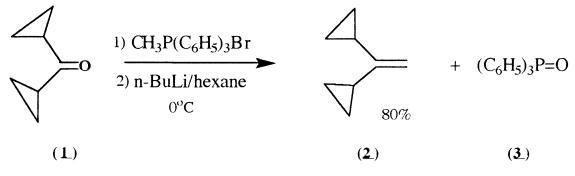
Synthesis
Methyltriphenylphosphonium bromide (11.6 g, 32.4 mmoles)
was weighed into a dry, 250 mL three neck, round bottom flask, and
50 mL of dry THF (distilled from Na/benzophenone) was cannulated
into the reaction vessel. A positive pressure of argon was maintained
throughout the reaction. The suspension was cooled to 0-5 ??, and
n-butyllithium, 2.5 M in hexane (14.0 mL, 32.4 mmoles) added
dropwise from an addition funnel to the well-stirred solution. A
yellow color developed upon addition of the base, indicative of
formation of the Wittig reagent.
Dicyclopropyl ketone (1) (3.5 mL, 32.4 mmoles), cooled to O??, a bottle with vacuum distilled dicyclopropyl ketone was kept in ice-water bath for 30 minutes, then
(1) was added dropwise to the Wittig reagent. The color changed
from yellow to off-white once the addition was complete. The
mixture was allowed to warm slowly to room temperature. The
reaction mixture was stirred overnight under a positive pressure of
argon.
After 24 hr, the reaction mixture was extracted successively
with 25 mL of water and 25 mL of saturated NaCI solution. The
organic layer was dried over anhydrous MgSO4. The solvent was
removed by fractional distillation using a short Vigreux column, and
the residue analyzed by gas chromatography (Hewlett Packard 5890,
using a DB-5 column, FID detector, 30 m length, 0.53 mm id). The
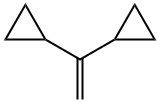
reaction mixture consisted of 1,1-dicyclopropylethylene (2) (80%),
unreacted dicyclopropyl ketone (10%), and unidentified compounds
(10%). Triphenylphosphine oxide (3) was the primary byproduct .