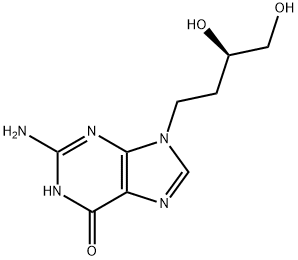
Manufacturing Process
Preparation of R-(+)-9-(3,4-dihydroxybutyl)guanine:
Step A: preparation of R-(+)-1,2,4-butantriol:
R-(+)-Dimethyl maleate (1.62, 10 mmol), prepared according to Boger, D.L.
and Panek, J.S., J. Org. Chem. 1981, 46, 1208-10, was dissolved in THF (10
ml) and added dropwise to a prewarmed suspension of lithium aluminium
hydride (0.63 g, 16.5 mmol) in THF (15 ml). The reaction mixture was stirred
overnight at 55°C. After sequential addition of water (0.62 ml), 10% sodium
hydroxide (1.20 ml) and water (1.90 ml) the mixture was filtered and the
solid residue was boiled twice with THF (2x20 ml) and filtered. The combined filtrates were pooled and evaporated under reduced pressure (30°C) leaving
crude 1,2,4-butantriol (0.7 g, 6.6 mmol) 66%.
Step B: preparation of R-(+)-isopropylidenbutan 1,2,4-triol:
R-(+)-1,2,4-butantriol (0.7 g, 6.6 mmol), prepared as described in step (a)
above, was stirred for 1.5 hr in acetone (50 ml) containing 3 drops of conc.
perchloric acid a satured solution of sodium bicarbonate in water (5 ml) was
added and the stirring was continued for additional 10 min. The precipitate
was filtered off and the filtrate evaporated under reduced pressure [2.7
kPa,(20 mm Hg), 30°C]. The residue was taken up in ethyl acetate, washed
with satured aqueous sodium bicarbonate (5 ml) and brine (5 ml), and dried
over magnesium sulfate. Evaporation of the solvent and distillation gave the
title compound as a colourless oil (0.3 g, 2.05 mmol, 31%): b.p. 104°-
106°C/20 mm Hg; nD
20=1.4390.
Step C: preparation of R-(+)-4-bromo-isopropylidenebutan-1,2-diol:
R-(+)-Isopropylidene-butan-1,2,4-triol (0.3 g, 2.05 mmol) and
triphenylphosphine (0.63 g, 2.4 mmol) were dissolved in methylene chloride
(5 ml) and cooled to 0°C. N-Bromosuccinimide (0.38 g, 2.16 mmol) was
added in small portions with stirring at 0°C. After additional 1 hr of stirring at
0°C hexane (15 ml) was added and the resulting precipitate was removed by
filtration and washed twice with hexane (2x5 ml). The combined hexane
solution was passed through a short column of silica gel (5 g). Elution with
hexane (15 ml) gave after evaporation and distillation the title compound as a
colorless oil (0.2 g, 0.96 mmol, 47%): b.p. 74°-76°C/20 mm Hg,
nD
20=1.4630. [α]D
20=+27.7° (C=20, CHCl3).
Step D: preparation of R(+)-4-(2-amino-6-chloropurin-9-yl)isopropylidenebutane-
1,2-diol:
2-Amino-6-chloropurin (162 mg, 0.96 mmol), R-(+)-4-bromo-isopropylidenebutandiol
(200 mg, 0.96 mmol) and potassium carbonate (132 mg) was
mixed in DMF (10 ml). After stirring for 16 hr the reaction mixture was filtered
through celite and the solvent evaporated under reduced pressure [13 Pa (0.1
mm Hg), 50°C]. The residue was triturated with warm chloroform (5 ml) and
undissolved material was filtered off. Evaporation of the solvent gave a pale
yellow crystalline solid consisting mainly of the 9- and 7-isomers. These were
separated by silica gel flash chromatography. Elution with
chloroform/methanol (15:1) gave the title compound in pure form (106 mg,
0.36 mmol, 37%): MP: 129°-130°C, [α]D
21 =+57.5° (C=6.97, CHCl3).
Step E: preparation of R-(+)-9-(3,4-dihydroxybutyl)guanine:
R-(+)-4-(2-Amino-6-chloropurin-9-yl)isopropylidene-butane-1,2-diol (100 mg,
0.33 mmol) prepared according to step (d) above was dissolved in
hydrochloric acid (1 mol/L) and refluxed for 1 hr. The solution was
concentrated in vacuum and the residue dissolved in water (5 ml) and made
alkaline by addition of aqueous ammonium hydroxide. After evaporation the
solid residue was recrystallized from water giving the title compound as white
needles (40 mg, 0.17 mmol, 51%). [α]D
21=+30.8° (C=0.25, water). (+/-)-
Form had MP: 260°-261°C.