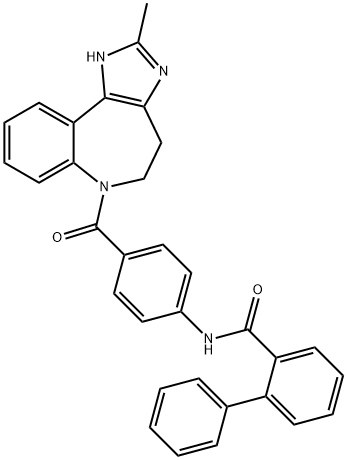
CONIVAPTAN
- Product NameCONIVAPTAN
- CAS210101-16-9
- CBNumberCB51011161
- MFC32H26N4O2
- MW498.583
- MDL NumberMFCD09838361
- MOL File210101-16-9.mol
- MSDS FileSDS
CONIVAPTAN Price
| Product number | Packaging | Price | Product description | Buy |
|---|---|---|---|---|
| Biosynth Carbosynth FC159956 | 10mg | $70 | Conivaptan |
Buy |
| Biosynth Carbosynth FC159956 | 25mg | $125 | Conivaptan |
Buy |
| Biosynth Carbosynth FC159956 | 50mg | $200 | Conivaptan |
Buy |
| Biosynth Carbosynth FC159956 | 100mg | $300 | Conivaptan |
Buy |
| Biosynth Carbosynth FC159956 | 250mg | $400 | Conivaptan |
Buy |
CONIVAPTAN Chemical Properties,Usage,Production
Description
Conivaptan, a vasopressin antagonist, was discovered and developed by Yamanouchi for the treatment of hyponatraeum associated with congestive heart failure.Uses
2-Methyl-1,4,5,6-tetrahydrobenzo[b]imidazo[4,5-d]azepine is an impurity of Conivaptan(C384700). Conivaptan is used in the treatment of congestive heart failures.Definition
ChEBI: The amide resulting from the formal condensation of 4-[(biphenyl-2-ylcarbonyl)amino]benzoic acid with the benzazepine nitrogen of 2-methyl-1,4,5,6-tetrahydroimidazo[4,5-d][1]benzazepine. It is an antagonist for two of the three types of argini e vasopressin (AVP) receptors, V1a and V2. It is used as its hydrochloride salt for the treatment of hyponatraemia (low blood sodium levels) caused by syndrome of inappropriate antidiuretic hormone (SIADH).Synthesis
After looking at several different approaches to the synthesis, a convergent approach, shown in the Scheme 4, was developed for large scale synthesis. Bromination of benzazepinone 10 with pyridinium hydrobromide perbromide in chloroform followed by recrystallization gave bromide 11. Reaction of bromide 11 with ethaneimidate hydrochloride in the presence of potassium carbonate in toluene or chloroform gave the desired imidazole 12 in 69% yield. Although chlo-roform provided a slightly better yield, for large scale preparation, toluene was used to minimize halogenated solvent waste and because the quality of product was similar or better than with use of chloroform. Deprotection of the tosylate was found to be effective with heating the sulfonamide 12 in 80% sulfuric acid at 80oC. The benzazepinone product 13 was obtained in 90% yield after crystallization from acetonitrile and water mixture.Synthesis of the coupling partner 16 required to provide conivaptan was synthesized in 95% yield from biphenyl 2- benzoic acid via sequential reaction with thionyl chloride in toluene followed by coupling with aminobenzoic acid in acetone with N,N-dimethylaniline as a base. High quality acid 16 was obtained by crystallization from DMF and water. The acid 16 was activated by converting it into acid chloride with thionyl chloride in acenonitrile, to which was added imidazo benzazepine 13 in toluene and, after recrystallization in acidic ethanol, gave conivaptan hydrochloride (III) in 90% yield.

Preparation Products And Raw materials
CONIVAPTAN Supplier
Global(39)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| 0086-13720134139 | candy@biochempartner.com | CHINA | 965 | 58 | |
| 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 | |
| +86-0371-86658258 +8613203830695 |
factory@coreychem.com | China | 29809 | 58 | |
| +1-781-999-5354 +1-00000000000 |
marketing@targetmol.com | United States | 32160 | 58 | |
| 0592-5800732; +8613806035118 |
xie@china-sinoway.com | China | 988 | 58 | |
| +8618327326525 | masar@topule.com | China | 8467 | 58 | |
| +86-852-30606658 | market18@leapchem.com | China | 43340 | 58 | |
| +86-18791163155 +86-13119157289 |
13119157289@163.com | China | 3558 | 58 | |
| +8613720134139 | orders@jknbiochem.com | China | 5221 | 58 | |
| 571-89925085 | sales@amadischem.com | China | 131957 | 58 |
210101-16-9, CONIVAPTANRelated Search
PROMPT×
PROMPT
The What'sApp is temporarily not supported in mainland China
The What'sApp is temporarily not supported in mainland China
Cancel
Determine
