Synthesis
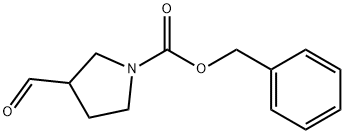
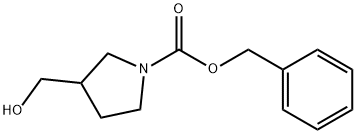
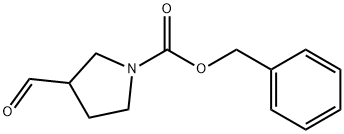
General procedure for the synthesis of 1-Cbz-3-pyrrolidinecarboxaldehyde from 1-Cbz-3-hydroxymethylpyrrolidine: To a solution of dichloromethane (5 mL) of oxalyl chloride (0.23 mL, 2.8 mmol) was added drop-wise dimethylsulfoxide (0.43 mL, 5.6 mmol) dissolved in dichloromethane (1 mL) at -75 °C and the reaction was stirred for 2 min. Subsequently, a solution of N-benzyloxycarbonyl-3-hydroxymethylpyrrolidine (0.56 g, 2.5 mmol) in dichloromethane (2 mL) was slowly added, and stirring was continued for 0.5 hr keeping the temperature at -75 °C. Upon completion of the reaction, triethylamine (1.8 mL, 12.8 mmol) was added and stirred at the same temperature for 5 minutes before slowly warming to room temperature. The reaction was quenched by the addition of water (10 mL) and the product was extracted with dichloromethane (100 mL). The organic phase was washed sequentially with water (100 mL) and saturated brine (100 mL) and dried over anhydrous sodium sulfate. The solvent was removed by concentration under reduced pressure and the residue was purified by silica gel column chromatography (eluent: ethyl acetate/hexane, 1:1, v/v) to afford the target product 1-Cbz-3-pyrrolidinecarboxaldehyde (0.46 g, 78% yield). The product was characterized by 1H NMR (200 MHz, CDCl3): δ 9.7 (s, 1H), 7.42-7.28 (m, 5H), 5.14 (s, 2H), 3.90-3.72 (m, 1H), 3.68-3.35 (m, 3H), 3.15-2.96 (m, 1H), 2.32-2.02 (m, 2H).
References
[1] Bioorganic and Medicinal Chemistry Letters, 2007, vol. 17, # 8, p. 2150 - 2155
[2] Patent: WO2005/26154, 2005, A1. Location in patent: Page/Page column 110
[3] Patent: WO2005/26165, 2005, A1. Location in patent: Page/Page column 137