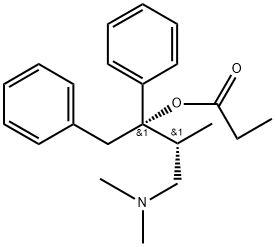
Definition
ChEBI: The (1S,2R)-(+)-diastereoisomer of propoxyphene.
brand name
Darvon-N (Xanodyne).
Biological Functions
Propoxyphene (dextropropoxyphene; Darvon) is structurally
related to methadone but is much less potent as
an analgesic. Compared with codeine, propoxyphene is
approximately half as potent and is indicated for the
treatment of mild pain. It is not antipyretic or antiinflammatory
like aspirin and is less useful than aspirin in
most cases of mild pain. Toxicity from propoxyphene,
especially in combination with other sedatives, such as
alcohol, has led to a decrease in its use. Death following
ingestion of alcohol in combination with propoxyphene
can occur rapidly (within 20 minutes to 1 hour). The
drug is not indicated for those with histories of suicide
or depressive illnesses.
Like meperidine, propoxyphene has an active
metabolite, norpropoxyphene, that is not analgesic but
has excitatory and local anesthetic effects on the heart
similar to those of quinidine. Use of the drug during
pregnancy is not safe.Teratogenic effects have been observed
in newborns, as have withdrawal signs at birth.
As with morphine, propoxyphene requires adequate hepatic
and renal clearance to prevent toxicity and drug
accumulation. It is thus contraindicated in the elderly
patient and those with renal or liver disease.
Propoxyphene interacts with several drugs. The use
of sedatives in combination with propoxyphene can be
fatal. In addition, the metabolism of the drug is increased
in smokers due to induction of liver enzymes.
Thus, smokers may require a higher dose of the drug for
pain relief. Propoxyphene enhances the effects of both
warfarin and carbamazepine and may increase the toxicity
associated with both drugs, such as bleeding and sedation,
respectively.
The abuse liability of propoxyphene is low because
of the extreme irritation it causes at the site of injection.
Oral use is the preferred route of administration for this
reason.
General Description
Most of the structural changes to the methadone skeletonresulted in compounds with decreased opioid potencies, thusmost of these compounds, with the exception of LAAM werenot developed. Propoxyphene is a derivative of methadonemarketed in 1957 as the enantiomerically pure (2S, 3R)-4-(Dimethylamino)-3-methyl-1,2,-diphenyl-2-butanol propionate(ester). It is only about 1/10th as potent as morphine asan analgesic yet retains all the same opioid adverse effects.One propoxyphene 65-mg capsule has the same analgesic effectof 650 mg of aspirin or 1,000 mg of acetaminophen, thusoverdoses of propoxyphene can occur if patients do not followthe prescribed dose. Between 1981 and 1999, 2,110 accidentaldeaths were reported because of propoxyphene.Propoxyphene and all propoxyphene combination productsare listed using the Beers criteria as medications to avoid inpatients older than 65 years of age. The metabolism ofpropoxyphene also contributes to the potential dangers of thedrug. Propoxyphene is metabolized via N-demethylation toform norpropoxyphene. Norpropoxyphene has been shownto build up in cardiac tissues and result in naloxone-insensitivecardiotoxicity. The weak analgesic action and potentialrisk to the patient have some health practitioners advocatingto remove all drugs containing propoxyphene from the market.The hydrochloride salt is marketed as Darvon, the napsylatesalt as Darvon-N, both salts are also available combinedwith acetaminophen (Darvocet, Darvocet-N) and apropoxyphene, aspirin, caffeine product is also available.
Safety Profile
Poison by ingestion,
intraperitoneal, subcutaneous, and
intravenous routes. Human systemic effects
by ingestion: change in cardiac rate,
respiratory depression, and coma. When
heated to decomposition it emits toxic
fumes of NOx.