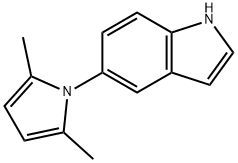
Synthesis
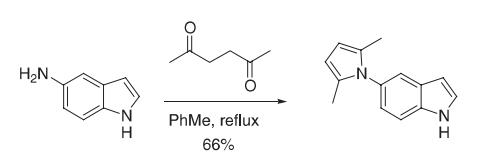
A mixture of 5-aminoindole (120.0 g, 0.908 mol), acetonylacetone (200.0 mL, 1.70 mol), and toluene (400 mL) was heated at reflflux under nitrogen using a DeanStark trap for 6 h. The reaction was cooled and then poured through a silica gel fifilter (~ 2 kg) followed fifirst by hexanes (4 L) and then by 6% ether in hexanes to afford 133.3 g of a pink solid. Recrystallization of this solid in ether/hexanes afforded 126.1 g (66%) of the 2,5-dimethylpyrrole as an off-white solid. Reference: Macor, J. E.; Chenard, B. L.; Post, R. J. J. Org. Chem. 1994, 59, 7496−7498.