Chemical Properties
Chlorinated rubber is typically prepared by treating a solution of masticated
natural rubber in chloroform or carbon tetrachloride with chlorine at
80-100°C until sampling indicates the product has a chlorine content of
about 65%. During this time, hydrogen chloride is evolved. After the passage
of chlorine has been stopped, the solution is refluxed until the evolution of hydrogen chloride ceases; this results in a product of good stability. The
chlorinated rubber is then isolated by precipitation with methanol.
Definition
An elastomer (natural
rubber or a polyolefin) to which 65% of chlorine
has been added to give a solid, film-forming resin.
White, amorphous powder available in viscosity
grades from 5 to 125 c P, the figures indicating vis-
cosity of a 20% solution in tol
Preparation
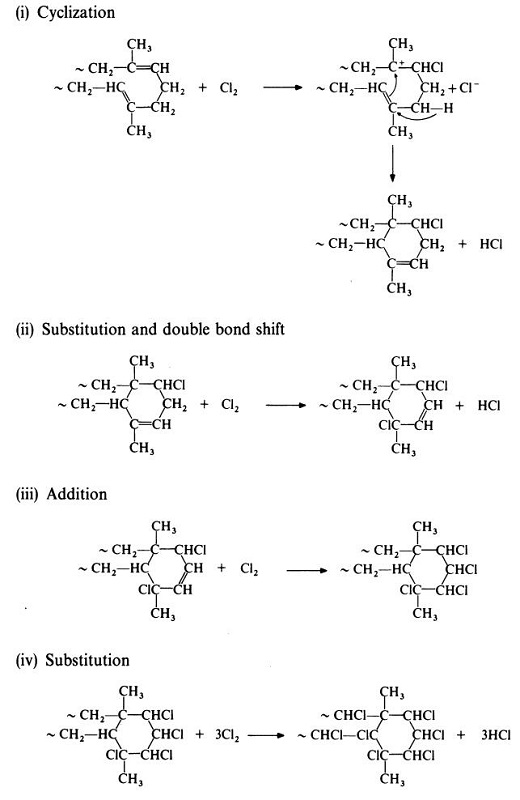
The reaction of natural rubber and chlorine is complex and possibly
involves the following sequence£o
Hazard
Do not dry-mill chlorinated rubber with
zinc oxide; mixture reacts violently at 216C. Do
not use in baked enamels.



