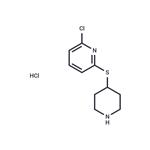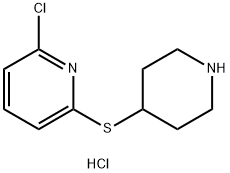
Anpirtoline hydrochloride
- Product NameAnpirtoline hydrochloride
- CAS99201-87-3
- CBNumberCB43037142
- MFC10H14Cl2N2S
- MW265.2
- MOL File99201-87-3.mol
Chemical Properties
| storage temp. | Store at -20°C |
| solubility | Soluble in DMSO > 10 mM |
| form | White solid. |
| color | Off-white to light yellow |
Anpirtoline hydrochloride Price
| Product number | Packaging | Price | Product description | Buy |
|---|---|---|---|---|
| TRC A697853 | 10mg | $160 | AnpirtolineHydrochloride |
Buy |
| ApexBio Technology B6410 | 10mg | $266 | Anpirtolinehydrochloride |
Buy |
| ApexBio Technology B6410 | 50mg | $1127 | Anpirtolinehydrochloride |
Buy |
Anpirtoline hydrochloride Chemical Properties,Usage,Production
Originator
Anpirtoline Hydrochloride,YQBio (Shanghai) Co. Ltd.Uses
Anpirtoline Hydrochloride is a SR-1B agonist.Manufacturing Process
2-Chloro-6-(4-piperidinylthio)pyridine:The reaction is carried out under an argon atmosphere. 0.27 g of 80% sodium hydride (0.009 mol) are suspended in 10 ml of dimethylacetamide; the mixture is cooled with ice and then 0.615 g (0.004 mol) of solid 4- mercaptopiperidine hydrochloride are added and stirred for 10 minutes. A solution of 0.588 g (0.004 mol) of 2,6-dichloropyridine in 5 ml of dimethylacetamide are then added dropwise to this mixture and the reaction mixture is stirred for 2.5 hours at room temperature.
Working up of the reaction mixture: 25 ml of water are added dropwise with cooling, 20 ml of methylene chloride are then added, the organic phase is separated off, the aqueous phase is then extracted twice with 15 ml of methylene chloride each time, the combined organic phase is washed twice, in each case with 10 ml of water, dried with sodium sulfate, the solution is concentrated on a rotary evaporator, the residue is mixed with 10 ml of absolute ethanol and then reconcentrated.
The product which is obtained on removal of the eluant is diluted with 10 ml of ether, an equivalent quantity of HCl in isopropanol is added dropwise and the mixture is placed for several hours in a deep freezer after addition of seed crystals. The hydrochloride of the 2-chloro-6-(4-piperidinylthio)pyridine which crystallizes out is filtered off with suction, washed with ether and dried under oil pump vacuum at 50°C. Melting point of the hydrochloride 132°-133°C.
Therapeutic Function
Analgesic, Antidepressantstorage
Desiccate at +4°CPreparation Products And Raw materials
Raw materials
Anpirtoline hydrochloride Suppliers
Global(15)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| +1-781-999-5354 +1-00000000000 |
marketing@targetmol.com | United States | 32161 | 58 | |
| +1-+1(833)-552-7181 | sales@aladdinsci.com | United States | 57505 | 58 | |
| 888-539-0666 | info@emmx.com | United States | 8447 | 60 | |
| 021-65675885 18964387627 |
info@efebio.com | China | 9804 | 58 | |
| -- | info@bocsci.com | USA | 0 | 65 | |
| 4008210725 4008210725 |
malulu@leyan.com | China | 54987 | 58 | |
| 021-69568360 18916172912 |
order@med-bio.cn | China | 8140 | 58 | |
| 0519-85524369 | 3477467573@qq.com | China | 8617 | 58 | |
| 4008200310 | marketing@tsbiochem.com | China | 24644 | 58 | |
| 0513-66337626 18051384581 |
sales@chemhifuture.com | China | 5127 | 58 |
99201-87-3, Anpirtoline hydrochlorideRelated Search
PROMPT×
PROMPT
The What'sApp is temporarily not supported in mainland China
The What'sApp is temporarily not supported in mainland China
Cancel
Determine