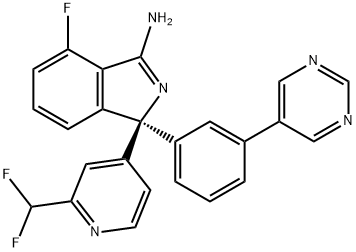
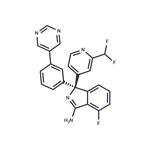
Uses
AZD3839 is an orally available, selective, reversible inhibitor of the β-site amyloid precursor protein cleaving enzyme BACE1 that can cross the blood-brain barrier. AZD3839 inhibits recombinant human BACE1 with a Ki=26.1 nM. AZD3839 inhibits A40 production in SH-SY5Y cells with an IC50 of 4.8 nM. AZD3839 binds to BACE1 and reduces the Aβ amyloid produced by the cleavage of amyloid precursor protein (APP) by BACE1 and γ-secretase. AZD3839 can be used in the field of Alzheimer's disease research[1][2][3].
Biological Activity
AZD3839 is a potent and selective BACE1 inhibitor (Ki = 26.1 nM/BACE1, 372.4 nM/BACE2, >25 μM/Cathepsin D by cell-free TR-FRET; hERG IC50 = 4.8 μM) th at effectively suppresses cellular BACE1 activity in neuron cultures (sAPPβ release IC50 = 16.7 nM/SH-SY5Y; Aβ40 release IC50 = 4.8 nM/SH-SY5Y AAP695wt, 32.2 nM/N2A, 24.8 nM/guinea pig cortical neurons, 51 nM/murine cortical neurons) and exhibits in vivo Aβ40-, Aβ42-, sAPPβ-reducing efficacy (80 & 160 μmol/kg mouse, p.o.; 100 & 200 μmol/kg guinea pigs, p.o., 2.5 & 20 μmol/kg cynomolgus monkeys, i.v.) with good pharmacokinetic properties and oral availability.
in vivo
In the female C57BL/6 mouse model, the brain concentration of AZD3839 free base (80 μmol/kg, 160 μmol/kg; oral gavage; single dose) increases rapidly and reaches a peak at 0.5 h. The levels of Aβ40, Aβ42 and sAPP in the brain decreased in a dose- and time-dependent manner. The plasma Aβ40 level is also significantly reduced, and the high dose (160 μmol/kg) had a more significant effect and lasted longer[1].
AZD3839 free base (100 μmol/kg; oral gavage; twice a day; 7 days) inhibits the level and accumulation of Aβ40 in the mouse brain and plasma in the female C57BL/6 mouse model[1].
AZD3839 free base (100 μmol/kg, 200 μmol/kg; oral gavage; single dose) reduces the levels of Aβ40 and Aβ42 in plasma, brain and cerebrospinal fluid of guinea pigs in a concentration- and time-dependent manner in the male Dunkin-Hartley guinea pig model[1].
AZD3839 free base (5.5 μmol/kg, 20 μmol/kg; intravenous injection; single dose) significantly reduces the levels of Aβ40, Aβ42 and sAPP in the cerebrospinal fluid of 3-5 year old female cynomolgus monkeys[1].
| Animal Model: | Female C57BL/6 mice (11-14 weeks old)[1] |
| Dosage: | 80 μmol/kg (35 mg/kg), 160 μmol/kg (69 mg/kg), 100 μmol/kg (43 mg/kg) |
| Administration: | 80 μmol/kg (35 mg/kg), 160 μmol/kg (69 mg/kg) as a single dose; 100 μmol/kg (43 mg/kg) as repeated doses twice daily for 7 days (dissolved in 5% dimethylacetamide and 20% hydroxypropyl-β-cyclodextrin in 0.3 M gluconic acid, pH 3, or 0.3 M gluconic acid, pH 3 alone) |
| Result: | Treated with a single dose of 80 μmol/kg or 160 μmol/kg, the brain concentration of AZD3839 peaked at 0.5 h after dosing. Brain Aβ40 levels decreased by 30% (80 μmol/kg dose) or 50% (160 μmol/kg dose) versus vehicle at 1.5 h after dose, and returned to baseline after 4.5 h (80 μmol/kg) or 8 h (160 μmol/kg).
The levels of brain Aβ42 and sAPP followed the same pattern as Aβ40. Both doses reduced plasma levels of Aβ40 by 60% versus vehicle over a prolonged period.
At 8 h after administration, the inhibitory effect started to decline within the low dose group, while maximal efficacy was maintained within the high dose group.
When treated with 100 μmol/kg twice daily for 7 days, the effect on brain and plasma Aβ40 was comparable to a single administration, and no drug accumulation was observed. |
| Animal Model: | Male Dunkin-Hartley guinea pigs (4-9 weeks old) |
| Dosage: | 100 μmol/kg (43 mg/kg), 200 μmol/kg (86 mg/kg) |
| Administration: | Oral gavage; 100 μmol/kg (43 mg/kg), 200 μmol/kg (86 mg/kg) as a single dose (dissolved in 20% hydroxypropyl-β-cyclodextrin in 0.3 M gluconic acid, pH 3) |
| Result: | After a single dose of 100 μmol/kg or 200 μmol/kg, brain Aβ40 was reduced up to 8 h after the dose in animals receiving the higher dose (20-60% versus vehicle), while guinea pigs receiving the lower dose demonstrated a reduction at 1.5-4.5 h after dose (20-30% versus vehicle).
Reduced CSF Aβ40 levels by 50% at 3 h after 200 μmol/kg dose, and still reduced the levels were by 40% at 8 h after dose, although the reduction failed to reach statistical significance at this later time point.
|
| Animal Model: | Female cynomolgus monkeys (3-5 years old)[1] |
| Dosage: | 5.5 μmol/kg (2.4 mg/kg), 20 μmol/kg (8.6 mg/kg) |
| Administration: | Intravenous injection via an implanted cannula in the vena cava at a constant infusion rate of 10 ml/kg/h for 15 min; single dose; dissolved in 0.3 M gluconic acid, pH 3.89 |
| Result: | Despite a large variation in the basal CSF levels of Aβ40, Aβ42, and sAPP, an intravenous infusion of 20 μmol/kg AZD3839 significantly reduced the levels of Aβ40, Aβ42, and sAPP in CSF between 3 and 12 h after dose. The inhibitory effect on sAPP was more pronounced than the effect |
References
[1] Sparve E et al. Prediction and modeling of effects on the QTc interval for clinical safety margin assessment, based on single-ascending-dose study data with AZD3839. J Pharmacol Exp Ther. 2014 Aug;350(2):469-78. DOI:
10.1124/jpet.114.215202[2] Jeppsson F et al. Discovery of AZD3839, a potent and selective BACE1 inhibitor clinical candidate for the treatment of Alzheimer disease. J Biol Chem. 2012 Nov 30;287(49):41245-57. DOI:
10.1074/jbc.M112.409110[3] Eketj?ll S, et al. AZD3293: A Novel, Orally Active BACE1 Inhibitor with High Potency and Permeability and Markedly Slow Off-Rate Kinetics. J Alzheimers Dis. 2016;50(4):1109-23. DOI:
10.3233/JAD-150834