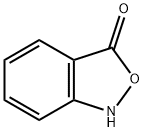
Synthesis
The mixture of 13.1 mg (0.078 mmol) of 2-azidobenzoic acid and
10.8 mg (0.078 mmol) of potassium carbonate in 10 mL ethanol was irradiated for 1 h
with intensive stirring. The reaction mixture was added to 50 mL water, extracted with
benzene and eluted through silica gel. The benzisoxazole-containing solution was
evaporated in vacuo affording 2,1-benzisoxazole-3(1H)-one with 75% yield. Pale
yellow crystals reddening at room temperature and stored at −20 °C. 1H NMR (400
MHz, CDCl3) δ 8.69 (s, 1H), 7.85 (d, J = 7.8 Hz, 1H), 7.68 (t, J = 7.7 Hz, 1H), 7.31 (s,
1H), 7.23 (d, J = 8.2 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ 169.0, 155.6, 135.2,
125.8, 124.7, 112.6, 111.9; IR (suspension in nujol oil, cm-1
): 3127 (ν NH); 1744,
1719 (ν C=O); 1068 (ν CO); m/z (rel, intensity) 136.02(M+
+2, 5.64), 134.91(M+
,
100.00), 118.93(2.73), 103.94(3.61), 90.92(M+-CO2, 14.07), 78.94(7.04), 76.97(5.45),
75.94(8.75), 63.98(10.01), 62.96(6.98), 51.99(11.24), 50.97(3.55), 49.95(4.86),
43.99(8.23), 39.95(2.83).