Manufacturing Process
A mixture of 2-methyl-5-trifluoromethylbenzothiazole (1 mole-equivalent), N-
bromosuccinimide (1 mole-equivalent), carbon tetrachloride (700 ml) and a
catalytic amount of benzoyl peroxide (0.2 g) was refluxed under irradiation by
an UV lamp for 14 hours. The reaction mixture was cooled to room
temperature, filtered to remove the precipitated succinimide and the filtrate
was evaporated to dryness. The resulting solid was chromatographed over
silica gel to obtain the 2-bromomethyl-5-trifluoromethyl-benzothiazole.
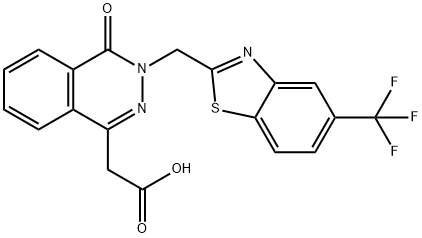
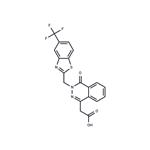
To a mixture of ethyl 4-oxo-3H-phthalazin-1-ylacetate (1 mole-equivalent) and
sodium hydride (50% w/w dispersion in mineral oil) in dimethylformamide
(150 ml) was added 2-bromomethyl-5-trifluoromethylbenzothiazole (1 mole-
equivalent) and the resulting mixture stirred at room temperature for 1 hour.
This reaction mixture was poured over ice-water (500 ml); sufficient 10% HCl
was added to adjust the pH to about 4.0 and the precipitated crude solid was
collected. This was chromatographed over silica gel to obtain the ethyl[4-oxo-
3-(5-trifluoromethylbenzothiazol-2-yl)-3,4-dihydro-phthalazin-1-yl]acetate;
MP: 134°-136°C.
A solution of methyl or ethyl [4-oxo-3-(5-trifluoromethylbenzothiazol-2-yl)-
3,4-dihydrophthalazin-1-yl]acetate (1 mole-equivalent) in methanol (50 ml)