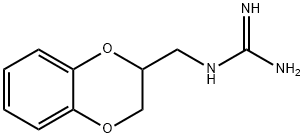
Guanoxan
- Product NameGuanoxan
- CAS2165-19-7
- CBNumberCB41117509
- MFC10H13N3O2
- MW207.23
- MOL File2165-19-7.mol
Chemical Properties
| Melting point | 164-165° |
| Boiling point | 339.3±45.0 °C(Predicted) |
| Density | 1.40±0.1 g/cm3(Predicted) |
| pka | pKa 12.3 (Uncertain) |
| FDA UNII | 9V0MRL0R5Y |
| ATC code | C02CC03 |
