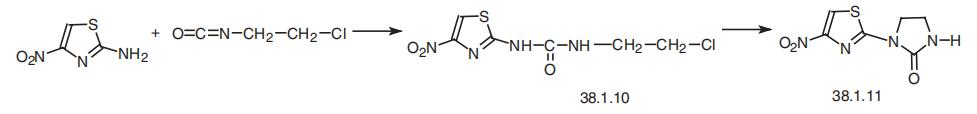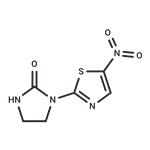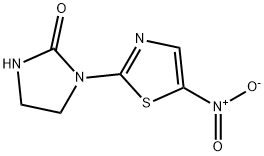
niridazole
- Product Nameniridazole
- CAS61-57-4
- CBNumberCB3896840
- MFC6H6N4O3S
- MW214.204
- EINECS200-512-6
- MDL NumberMFCD00128262
- MOL File61-57-4.mol
Chemical Properties
| Melting point | 260-262° |
| Density | 1.561 (estimate) |
| refractive index | 1.6200 (estimate) |
| form | solid |
| pka | 12.73±0.20(Predicted) |
| color | Yellow crystals from DMF/MeOH |
| Water Solubility | 0.13g/L(25 ºC) |
| FDA UNII | N116U8Y5QQ |
| IARC | 2B (Vol. 13, Sup 7) 1987 |
| Proposition 65 List | Niridazole |
| ATC code | P02BX02 |
| EPA Substance Registry System | Niridazole (61-57-4) |
Safety
| Hazardous Substances Data | 61-57-4(Hazardous Substances Data) |
| Toxicity | TDLo orl-rat: 8 g/kg/90W-I:NEO CALEDQ 4,305,78 orl-rat LD50:900 mg/kg FAZMAE 17,108,73 |