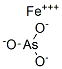Chemical Properties
Brownish-yellow powder. Soluble in
acids; insoluble in water. Nonflammable.
Uses
Combined with ammonium citrate (ferric
ammonium citrate) and used in medicine.
Definition
A
basic salt of variable composition.
General Description
A yellow-brown powder. Insoluble in water. Toxic by ingestion and inhalation and is a strong irritant. Ferric arsenite is used in medicines.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Ferric arsenite has weak oxidizing or reducing powers. Redox reactions can however still occur. The majority of compounds in this class are slightly soluble or insoluble in water. If soluble in water, then the solutions are usually neither strongly acidic nor strongly basic. These compounds are not water-reactive.
Hazard
Toxic by ingestion and inhalation, strong
irritant.
Health Hazard
Highly toxic, may be fatal if inhaled, swallowed or absorbed through skin. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Containers may explode when heated. Runoff may pollute waterways.