Description
Bacampicillin was synthesized by Ekstrom¨ et al. of Astra Lakemedel in 1975. It is ¨ more stable against acid than ampicillin and more rapidly absorbed orally; its absorption is less affected by a patient’s most recent meal than is the case for ampicillin. Bacampicillin is hydrolyzed by intestinal esterase after oral administration and then behaves the same as ampicillin. In double-blind comparison studies, bacampicillin was shown to be as effective as ampicillin when administered at half the dose.
Chemical Properties
White or almost white powder or granules, hygroscopic.
Originator
Penglobe,Astra,W. Germany,1977
Uses
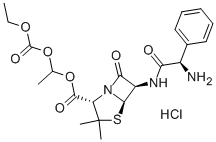
Bacampicillin is a penicillin class of antibiotic. Bacampicillin is a prodrug of ampicillin (A634300) with improved oral bioavailability.
Definition
ChEBI: The hydrochloride salt of bacampicillin.
Manufacturing Process
1'-Ethoxycarbonyloxyethyl 6-(D-α-azidophenylacetamido)penicillinate (98 g)
was prepared from sodium 6-(D-α-azidophenylacetamido)penicillinate (397 g,
1 mol), α-chlorodiethylcarbonate (458 g, 3 mols) and sodium bicarbonate
(504 g, 6 mols). The product showed strong IR absorption at 2090 cm-1 and
1780-1750 cm-1 showing the presence of azido group and β-lactam and ester
carbonyls.
It was dissolved in ethyl acetate (700 ml) and hydrogenated at ambient
conditions over a palladium (5%)on carbon catalyst (18 g). The catalyst was
removed by filtration and washed with ethyl acetate. The combined filtrates
were extracted with water at pH 2.5 by addition of dilute hydrochloric acid.
Lyophilization of the aqueous phase gave the hydrochloride of 1'-
ethoxycarbonyloxyethyl 6-(D-α-aminophenylacetarnido)penicillinate (94 g), MP
171°-176°C.
brand name
Spectrobid (Pfizer).
Therapeutic Function
Antibacterial
Clinical Use
Bacampicillin hydrochloride (Spectrobid) is the hydrochloridesalt of the 1-ethoxycarbonyloxyethyl ester of ampicillin.It is a prodrug of ampicillin with no antibacterial activity.After oral absorption, bacampicillin is hydrolyzed rapidly byesterases in the plasma to form ampicillin.
Oral absorption of bacampicillin is more rapid and completethan that of ampicillin and less affected by food.Plasma levels of ampicillin from oral bacampicillin exceedthose of oral ampicillin or amoxicillin for the first 2.5 hoursbut thereafter are the same as for ampicillin and amoxicillin.49 Effective plasma levels are sustained for 12 hours,allowing twice-a-day dosing.
Safety Profile
Poison by intraperitoneal andintravenous routes. Mildly toxic by ingestion andsubcutaneous routes. When heated to decomposition itemits very toxic fumes of NOx, SOx, and HCl.