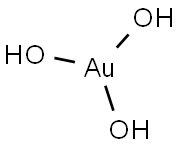
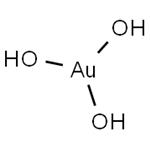
Chemical Properties
solid
Physical properties
Brown powder; decomposes at 100°C; insoluble in water; soluble in acid.
Uses
Synthesis of gold hydroxide complexes with
N-heterocyclic carbene has been reported. A variety of perfluoroaryls,
N-heterocyclic and alkynyl compounds may be synthesized by reacting gold(III) hydroxide with C–H and N–H compounds and arylboronic acids. Potassium aurate may be prepared by heating auric hydroxide with conc. KOH. 3
Uses
In gold-plating solutions; for decorating porcelains.
Production Methods
Gold(III) hydroxide is precipitated by mixing aqueous solutions of potassium auric chloride and sodium carbonate:2KAuCl4 + 3Na2CO3 + 3H2O → 2Au(OH)3 + 6NaCl + 2KCl + 3CO2
The product usually contains about three molecules of water of crystallization. It may alternatively be prepared by adding caustic soda solution to sodium auric cyanide: NaAu(CN)4 + 3NaOH → Au(OH)3 + 4NaCN.