Preparation
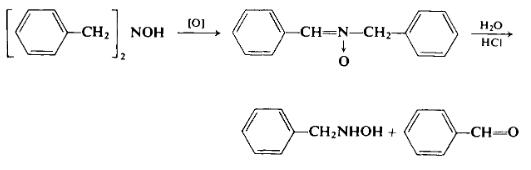
(a) Preparation of a-phenyl-N-benzylnitrone. To a flask equipped with a stirrer and condenser and containing 17.6 gm (0.0827 mole) of N,N-dibenzylhydroxylamine is added 36.8 gm (0.17 mole) of yellow mercuric oxide and 100 ml of ether. The mixture is stirred and the resulting exothermic reaction causes the ether to reflux for 1 hr. After a total of 3 hr reaction time the reaction mixture is filtered and concentrated to afford 15.7 gm (90%), m.p. 81.5-83.5°C (recrystallized from acetone-ligroin). Jones and Sneed [12a] earlier reported that air-KOH can also be used to effect this oxidation.
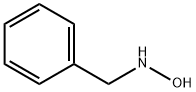
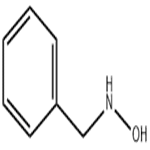
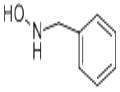
(b) Hydrolysis of a-phenyl-N-benzylnitrone to N-benzylhydroxylamine [12a]. To a flask containing 17.0 gm (0.0806 mole) of the above nitrone is added 34 ml of concentrated hydrochloric acid solution. Then the mixture is steam-distilled to remove the formed benzaldehyde. After the benzaldehyde is removed the hydrochloric acid is removed by heating over a low flame. To the cool residue is added a cold solution of sodium carbonate to neutralize the mixture. The mixture is filtered, and the filtrate, after being cooled to 0°C, is made alkaline. After 1 hr N-benzylhydroxylamine precipitates from the cold filtrate and is filtered to afford 6.0 gm (61%), m.p. 57°C (recrystallized from benzene-petroleum ether). Yields as high as 88% have been reported for this preparation.