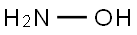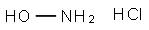Description
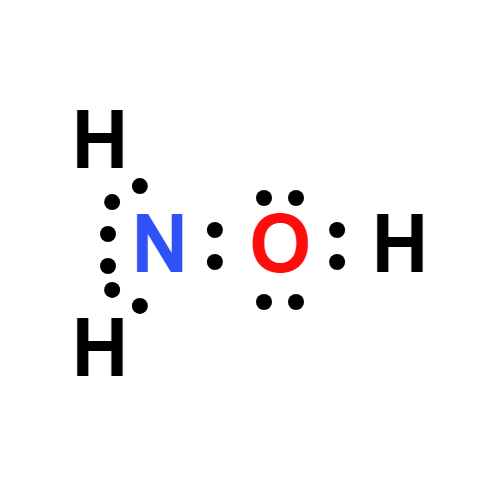
Hydroxylamine is the hydroxyl derivative of ammonia. Hydroxylamine is used as a nucleophile in aromatic substitution reactions and as a reducing agent. By the reaction with aldehydes, hydroxylamine forms oximes, which are intermediates in the commercial production of polyamide plastics. Some hydroxylamine converted oximes are used in smaller amounts as pharmaceuticals, pesticides, and varnishes to prevent formation of a skin. With it is reduction ability, hydroxylamine is used as an antioxidant in photographic developers, to stabilize polymerization monomers, and to reduce Cu2+ in the dyeing of acrylic fibers. Hydroxylamine can be converted into hydroxylamine-O-sulphonic acid, which is a good aminating agent. Hydroxylamine can also be used as an intermediate in nitrification. It is further used in semiconductor industry in the cleaning formations, such as for aluminum interconnect.
References
- Stephen A. Lawrence, Amines: Synthesis, Properties and Applications, 2004, ISBN 0521782848
- Howard Lees, Hydroxylamine as an intermediate in nitrification, Nature, 1951, vol. 169, 156-157
- Egon Wiberg and Nils Wiberg, Inorganic Chemistry, 2001, ISBN 0123526515
- https://www.britannica.com/science/hydroxylamine
- Karen A. Reinhardt and Richard F. Reidy, Handbook for Cleaning for Semiconductor Manufacturing: Fundamentals and Applications, 2011, ISBN 9780470625958
Description
Hydroxylamine was first synthesized by Wilhem Clemens
Lossen in 1865 in the laboratory of Wilhelm Heinrich Heintz
while working in Halle. The Lossen synthesis originally generated
hydroxylamine in aqueous solution. Anhydrous hydroxylamine
was prepared later by Lobry de Bruyn and Crismer in
1891. The free base is extremely volatile, and industrial-scale
production has been fraught with problems, including large
explosions at facilities in the United States and Japan. Much of
the hydroxylamine produced and transported is in salt form or
as a dilute aqueous solution.
Chemical Properties
slightly yellow liquid
Physical properties
White crystalline solid; orthogonal plates or needles; unstable; density 1.21g/cm3at 20°C; melts at 33°C; vaporizes at 58°C; very soluble in water, liquidammonia and lower alcohols; sparingly soluble in most other organic solvents;decomposes in hot water; pKa5.94 at 25°C.
Uses
Reducing agent used in photographic
processing, leather tanning, manufacturing of
nylon and other polymers; as a stabilizer for
natural rubber; to prevent the development of
objectionable tastes and odors during the refining
of fatty materials.
Uses
Reducing agent, organic synthesis.
Uses
Hydroxylamine is used as a reducing agent in photography, in
synthetic and analytical chemistry, as an antioxidant for fatty
acids and soaps, and as a dehairing agent for hides. In addition,
hydroxylamine is used in the production of cyclohexanone
oxime, an isomer of caprolactam, which is an intermediate in
the production of nylon-6. In the semiconductor industry,
hydroxylamine can be a component of a solution that dissolves
a photoresist following lithography. Hydroxylamine can also
be used to selectively cleave asparaginyl-glycine peptide bonds.
Definition
ChEBI: The simplest hydroxylamine, consisting of ammonia bearing a hydroxy substituent. It is an intermediate in the biological nitrification by microbes like bacteria.
Definition
hydroxylamine: A colourless solid,NH
2OH, m.p. 33°C. It explodes onheating and may be employed as anoxidizing agent or reducing agent. Itis made by the reduction of nitratesor nitrites, and is used in makingnylon. With aldehydes and ketones itforms oximes.
Preparation
Hydroxylamine is unstable as a free base. It is prepared from hydroxy-lamine hydrochloride, NH2OHHCl, which is obtained by electrolytic reduc-tion of ammonium chloride solution. The hydrochloride undergoes alkalinedecomposition to hydroxylamine, which is collected by vacuum distillation.
General Description
Odorless white crystalline solid. Sinks and mixes with water.
Air & Water Reactions
Decomposes rapidly at room temperature or when dissolved in hot water by internal oxidation-reduction. Reacts with water or steam to produce heat and corrosive liquids.
Reactivity Profile
HYDROXYLAMINE is a white solid, thermally unstable, decomposes rapidly at room temperature or when dissolved in hot water by internal oxidation-reduction. HYDROXYLAMINE should be stored below 10° C [Bailar, 1973, vol. 2, p. 272]. Explosive reaction with strong oxidizers (chromium trioxide, potassium dichromate) or powdered zinc upon heat. Reaction with zinc or calcium produces explosive bishydroxylamides. HYDROXYLAMINE ignites on contact with cupric sulfate, alkali metals (sodium, potassium), oxidants (e.g., barium oxide, barium peroxide, lead dioxide, potassium permanganate, chlorine), phosphorus trichloride and pentachloride. HYDROXYLAMINE reacts vigorously with hypochlorites, pyridine, carbonyls [Sax, 9th ed., 1996, p. 1875]. On contact with organic materials in thin layer (e.g., crystals on filter paper), HYDROXYLAMINE may ignite spontaneously in air. HYDROXYLAMINE explodes when heated above 70° C [Brauer, 1963, vol. 1, p. 502]. During a distillation process, an explosion occurred. Potassium hydroxide is thought to be involved in the explosion. Employees in the plant complained of chest pains and suffered chemical burns. Five people were killed by the explosion.
Hazard
Decomposes rapidly at room temperature,
violently when heated, detonates in flame-heated
test tube. Irritant to tissue.
Health Hazard
INHALATION: Moderately toxic by inhalation and oral routes with the following symptoms possible: headache, vertigo, tinnitus, dyspnea, nausea and vomiting, cyanosis, proteinuria and hematuria, jaundice, restlessness, and convulsion. Methemoglobinemia has been reported. EYES: Corrosive - highly irritating. SKIN: Irritating or corrosive to skin. INGESTION: Moderately toxic by inhalation and oral routes with the following symptoms possible; headache, vertigo, tinnitus, dyspnea, nausea and vomiting, cyanosis, proteinuria and hematuria, jaundice, restlessness, and convulsion. Methemoglobinemia has been reported.
Contact allergens
Hydroxylamine and its salts are used in various
branches of industry, as reducing agents in color film
developers or as reagents in laboratories.
Potential Exposure
Potential Exposure:Mutagen.Those involved in chemical synthesis or use of hydroxyI-amine. Used as a reducing agent.
First aid
If this chemical gets into the eyes, remove any con-tact lenses at once and irrigate immediately for at least 15 min,occasionally lifting upper and lower lids. Seek medical atten-tion immediately. If this chemical contacts the skin, removecontaminated clothing and wash immediately with soap andwater. Seek medical attention immediately. If this chemicalhas been inhaled, remove from exposure, begin rescue breath-ing (using universal precautions, including resuscitation mask)if breathing has stopped and CPR if heart action has stopped.Transfer promptly to a medical facility. When this chemicalhas been swallowed, get medical attention. If victim is con-scious, administer water or milk. Do not induce vomiting.Note to physician: Treat for methemoglobinemia. Test urineformethemoglobinemia. Spectrophotometry may berequired for precise determination of levels of methemoglo-bin in urine.
Carcinogenicity
Carcinogenicity of hydroxylamine and
its salts has not been demonstrated. Several
studies have shown a decreased incidence of
spontaneous mammary tumors in mice exposed
to the sulfate and hydrochloride.3–7 There was
some indication of an increase in the incidence
of spontaneous mammary tumors when the
sulfate was administered to older animals
whose mammary glands were already well
developed.
Environmental Fate
The large-scale production and use of hydroxylamine may
result in its release to the environment through various waste
streams. Hydroxylamine will exist solely as a vapor in the
ambient atmosphere, and will be degraded in the atmosphere
by reaction with photochemically produced hydroxyl radicals;
the half-life for this reaction in air is estimated to be 18 h.
Abiotic degradation of hydroxylamine by photochemically
produced peroxy radicals is an important environmental fate
process in surface waters, with the half-life of the reaction
measured at approximately 2 h. An estimated bioconcentration
factor of 3 suggests that the potential for bioconcentration in
aquatic organisms is low. If released terrestrially, hydroxylamine
will most likely exist in its protonated form due to its
pKa of 5.94; the protonated form is nonvolatile. Koc estimates
of 14 for hydroxylamine suggest that it may have very high
mobility in soil.
storage
(1) Color Code- Yellow Stripe (strong reducingagent): Reactivity Hazard; Store separately in an area iso-lated from flammables, combustibles, or other yellow-codedmaterials. (2) Color Code- Blue: Health Hazard/Poison:Store in a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Protect against physical damage. Store in cool, non-combustible buildings and separate from oxidizing materi-als. Open airtight containers occasionally to relieve pressurefrom decomposition products.
Shipping
Toxic solid, corrosive, inorganic, n.o.s. requires ashipping label of“POISONOUS/TOXIC MATERIALS,CORROSIVE.”It falls in DOT/UN Hazard Class 6.1 andPacking Group II.
Purification Methods
Crystallise it from n-butanol at -10o, collect it by vacuum filtration and wash it with cold diethyl ether. Harmful vapours. [Hurd Inorg Synth I 87 1939, Semon in Org Synth Coll Vol I 318 1932.]
Toxicity evaluation
Hydroxylamine acts as a reducing agent when absorbed
systemically, producing methemoglobin and the formulation
of Heinz bodies in the blood. It can induce hemolytic anemia.
It inhibits platelet aggregation and is a nitric oxide vasodilator.
Oxylamines such as hydroxylamine and methoxylamine
disturb DNA replication and act as potent mutagens, causing
nucleotide transition from one purine to another or one
pyrimidine to another.
Allergic reactions of the skin following dermal exposure to
hydroxylamine resemble contact eczema, or possibly urticaria
of Quincke’s edema. The pathogenesis of this reaction appears
to be mediated by a delayed type T-lymphocyte reaction.
Incompatibilities
Self reactive. Contaminants, tempera-tures above 129.4℃, or open flame can cause explosivedecomposition, especially in the presence of moisture andcarbon dioxide. Incompatible with strong acids, organicanhydrides, isocyanates, aldehydes, sodium, finely dividedzinc, some metal oxides. Aqueous solution is a weak base.Contact with strong oxidizers may cause a fire and explo-sion hazard. Attacks some metals. Contact with calcium orzinc forms a heat-sensitive explosive [bis(hydroxylamide)](Sax).