Description
Uracil mustard appears as creamy/off-white, odourless, crystalline powder. It is used as an
anti-cancer medicine. Uracil mustard is a chemotherapy drug that belongs to the class of
alkylating agents. It is used for its anti-neoplastic properties. It works by damaging deoxyribonucleic
acid (DNA), primarily in cancer cells that preferentially take up the uracil due
to their need to make nucleic acids during their rapid cycles of cell division. At high concentrations
of the drug, cellular RNA and protein synthesis are also suppressed. The DNA
damage leads to apoptosis of the affected cells. Chemically it is a derivative of nitrogen
mustard and uracil. Uracil mustard is a non-combustible substance itself; it does not burn
but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidisers
and may ignite combustibles – wood, paper, oil, clothing, etc. Contact with metals
may evolve flammable hydrogen gas. Containers may explode when heated.
Originator
Uracil Mustard,Upjohn,US,1962
Definition
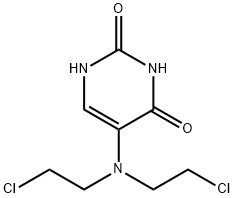
ChEBI: 5-[bis(2-chloroethyl)amino]uracil is a nitrogen mustard and an aminouracil.
Manufacturing Process
Preparation of 5-[bis(2-Hydroxyethyl)Amino] Uracil: 20 grams (0.157 mol) of
5-aminouracil was mixed with 350 ml of water, 23 ml of glacial acetic acid,
and 160 ml of ethylene oxide in a one-liter flask immersed in an ice bath. The
reaction mixture was stirred and allowed to come to room temperature slowly
(as the ice melted), and stirring was continued for two days. A clear solution
resulted to which was added 250 ml of water and 60 grams of Dowex-50 in
the acid form. The mixture was stirred for 15 minutes, and the resin was
collected on a filter. It was washed with water and the crude 5-[bis(2-
hydroxyethyl)amino] uracil was eluted with a 10% aqueous solution of
ammonium hydroxide. This eluate was evaporated to dryness, and the solid
that remained was heated with 350 milliliters of isopropyl alcohol.Undissolved substances were removed by filtration and the filtrate was
concentrated on a steam bath to a volume of about 125 ml and cooled to
effect crystallization. After 20 hours at room temperature the crystals that had
formed were recovered, washed with isopropyl alcohol, and dried, yielding
15.61 grams (46.2%) of crystalline 5-[bis(2-hydroxyethyl)amino] uracil having
a MP of 157° to 163°C. An analytical sample, obtained by several
recrystallizations from isopropyl alcohol, melted at 166° to 168°C.
Preparation of 5-[bis(2-Chloroethyl)Amino] Uracil: 13 ml of thionyl chloride
was added to 52 ml of diethylene glycol dimethyl ether accompanied by
stirring. Heat was generated, and sulfur dioxide and hydrogen chloride were
liberated. The mixture was cooled and 5.58 grams of 5-[bis(2-
hydroxyethyl)amino] uracil was added, followed by 8 ml of thionyl chloride, No
evidence of reaction was noted, and the reaction mixture was heated to about
40°C, gas then being evolved. After one hour at 40°C, 5 ml of thionyl chloride
was added, and after 30 minutes, another 3 ml was added. The mixture was
then heated to 55°C, whereupon it darkened and all of the solid dissolved.
After cooling and storage at room temperature for 20 hours, three volumes of
benzene was added and a dark solid precipitated. After one hour, the dark
solid was collected on a filter, washed with benzene, and dissolved in a
minimum of boiling methanol. Crystals formed upon cooling; and after 18
hours in the refrigerator, they were recovered on a filter, washed with cold
methanol, and dried under reduced pressure, yielding 2.96 grams of 5-[bis(2-
chloroethyl)amino] uracil. The product was recrystallized by dissolving in a
minimum of hot methanol and adding water until the solution became cloudy;
2.25 grams of 5-[bis(2-chloroethyl)amino] uracil was recovered after cooling
the mixture to 4°C for 16 hours (MP 200° to 205°C). A small sample was
recrystallized again, and it melted at 198° to 204°C.
Therapeutic Function
Cancer chemotherapy
General Description
Creamy white crystals or off-white powder. Used as an anti-cancer medicine.
Air & Water Reactions
Slightly soluble in water [Merck].
Reactivity Profile
URACIL MUSTARD (500 MG) (FOR U.S. SALE ONLY) reacts as a base.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Safety Profile
Suspected carcinogen withexperimental carcinogenic and neoplastigenic data. Adeadly poison by ingestion and intraperitoneal routes.Mutation data reported. When heated to decomposition itemits very toxic fumes of Cl?? and NOx.
Carcinogenicity
Uracil mustard was reportedly
carcinogenic in both mice and rats following multiple intraperitoneal
injections. It produced a dose-related increase in
lung tumor incidence in mice and tumors in a variety of other
organs in both mice and rats. The IARC reviewed the
preceding data and deemed it “sufficient evidence of carcinogenicity
in animals.” Based on this information, its mutagenic
potential, analogy to other nitrogen mustards, and a
lack of carcinogenicity data in humans, the IARC classified
uracil mustard in Group 2B (possibly carcinogenic to
humans). Uracil mustard compared to nitrogen mustard
itself in the same assay (lung tumor assay in strain A mice,
intraperitoneal dosing) was found to be more potent as a
tumorigen than nitrogen mustard. An in vitro assay to
predict carcinogenicity gave a positive response predicting
that uracil mustard would be a carcinogen in rodent test
models. The assay used focus formation in a stable bovine
papillomavirus type 1 DNA carrying a mouse fibroblast cell
line that does not require transfection, infection with virus,isolation of primary cells from animals, or addition of a
microsomal fraction.