Manufacturing Process
80 g of 1-methyl-piperidone-4 and 70 g of aniline are boiled, using a water
separator in 350 ml of toluene to which several drops of glacial acetic acid
have been added, until the theoretical quantity of water (12.7 ml) has
separated out. The toluene is then distilled off and the remains are
fractionated at reduced pressure. At a boiling point of 156°C/13 mm of Hg
pressure, 118 g of a weakly yellow colored oil of the anil of 1-methyl-1-
piperidone-4 are obtained.
100 g of the above described anil of 1-methyl-1-piperidone-4 are boiled for 8
hours, using a reflux condenser, with 30 g of activated borings of aluminum in
300 ml of methanol diluted with 60 ml of water. The liquid phase is then
separated from the solid phase, the solvent is evaporated and the residuum is
fractionated at reduced pressure, 95 g of a colorless oil being obtained boiling
at 163-165°C at 15 mm of Hg pressure. The oil solidifies at once to a mass of
crystals of 4-N-phenylaimno-1-methylpiperidine. After having been
recrystallized from dibutyl ether the base melts at 87°C, its dihydrochloride
has the melting point of 246°C.
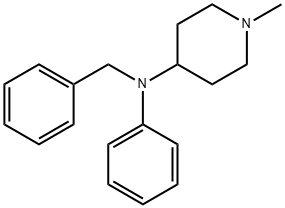
95 g of 4-N-phenylamino-1-methylpiperidine are boiled together with 22 g of
pulverized sodium amide in 300 ml of benzene, using a reflux condenser,
while nitrogen is passed through the reaction mixture, until the evolution of
ammonia has ceased. 64 g of benzyl chloride are then gradually added drop
by drop to the boiling reaction product and boiling is continued for several
hours. After having been cooled the solution is shaken with water and
subsequently dried with potassium carbonate. The solvent having been
evaporated the remaining base of 4-(N-phenyl-N-benzyl)-amino-1-
methylpiperidine solidifies with the formation of crystals, the yield amounting
to 123 g. The base is recrystallized from dibutyl ether and has a melting point
of 115°C; its dihydrochloride melts at 189°C.