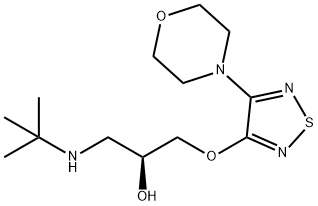
Definition
ChEBI: (S)-timolol (anhydrous) is the (S)-(-) (more active) enantiomer of timolol. A beta-adrenergic antagonist, both the hemihydrate and the maleate salt are used in the mangement of glaucoma, hypertension, angina pectoris and myocardial infarction, and for the prevention of migraine. It has a role as an antiglaucoma drug, an antihypertensive agent, an anti-arrhythmia drug and a beta-adrenergic antagonist. It is a conjugate base of a (S)-timolol(1+). It is an enantiomer of a (R)-timolol.
Manufacturing Process
Step A: Preparation of 3-tert-Butylamino-2-Oxopropanol - To an aqueous
solution of tert-butylamine (1 mol) at ambient temperature, there is added
slowly and with vigorous stirring 2 mols bromoacetol. The reaction mixture is
allowed to stand at ambient temperature for about 5 hours whereupon it is
made basic by the addition of sodium hydroxide.
The reaction mixture then is extracted with ether, the excess amine is
removed from the ethereal solution under reduced pressure and the ether
then removed by evaporation to give 3-tert-butylamino-2-oxopropanol.
Step B: A solution of the 3-tert-butylamino-2-oxopropanol in a mixture of
pyridine hydrochloride and pyridine is treated with p-toluenesulfonylchloride.
The mixture is stirred for 1/2 hour at 25° to 30°C and then poured into cold water. The solution is treated with potassium carbonate and the pyridine
evaporated in vacuo at a temperature between 55° and 60°C. The aqueous
residue is treated with potassium carbonate and the mixture extracted with
methylene chloride. Evaporation of the dried extract provides 1-
toluenesulfonyloxy-2-oxo-3-tert-butylaminopropane.
Step C: Preparation of 3-Morpholino-4-(3-tert-Butylamino-2-Oxopropoxy)-
1,2,5-Thiadiazole - The 1-toluenesulfonyloxy-2-oxo-3-tert-butylaminopropane,
prepared as described in Step B, (11 mols) is added to 0.80 N methanolic
sodium methoxide (15 ml) at 0°C. The mixture is stirred for 15 minutes at 0°
to 5°C, treated with 3-morpholino-4-hydroxy-1,2,5-thiadiazole (4.29 grams)
and then refluxed for 16 hours. The solvent is evaporated in vacuo and the
residue is treated with excess potassium carbonate to provide 3-morpholino-
4-(3-butylamino-2-oxopropoxy)-1,2,5-thiadiazole.
Step D: Chemical Reduction Preparation of 3-Morpholino-4-(3-tert_x0002_Butylamino-2-Hydroxypropoxy)-1,2,5-Thiadiazole - The 3-morpholino-4-(3-
tert-butylamino-2-oxopropoxy)-1,2,5-thiadiazole (0.01 mol) is dissolved in
isopropanol (10 ml). To the solution is added sodium borohydride in portions
until the initial evolution of heat and gas subsides. The excess sodium
borohydride is destroyed by addition of concentrated hydrochloric acid until
the mixture remains acidic. The precipitate of sodium chloride is removed,
ether is added, and the solution is concentrated to crystallization. The solid
material is removed by filtration and dried thus providing 3-morpholino-4-(3-
tert-butylamino-2-hydroxypropoxy)-1,2,5-thiadiazole, MP 161° to 163°C (as
hydrochloride).
Alternative Step D: Reduction with a Reductate - Sucrose (1 kg) is dissolved
in water (9 liters) in a 20-liter bottle equipped with a gas trap. Baker's yeast
(Saccharomyces cerevisiae, 1 kg) is made into a paste with water (1 liter) and
added to the sucrose solution with stirring. After lively evolution of gas begins
(within 1 to 3 hours), 3-morpholino-4-(3-tert-butylamino-2-oxopropoxy)-
1,2,5-thiadiazole hydrogen maleate [1.35 mols, prepared by reaction of the 3-
morpholino-4-(3-tert-butylamino-2-oxopropoxy)-1,2,5-thiadiazole with an
equimolar quantity of maleic acid in tetrahydrofuran]. The mixture is allowed
to stand until fermentation subsides, after which the bottle is kept in a 32°C
incubator until all fermentation has ended (in approximately 1 to 3 days). The
yeast is filtered off with addition of diatomaceous earth and the filtrate is
evaporated to dryness to give S-3-morpholino-4β-tert-butylamino-2-
hydroxypropoxy)-1,2,5-thiadiazole, MP 195° to 198°C (as hydrogen maleate),
according to US Patent 3,619,370.
Step E: The base may be converted to the maleate by maleic acid
Metabolism
Timolol (Timoptic) is almost completely absorbed
from the gastrointestinal tract. Peak plasma levels occur
2 to 4 hours after oral administration; the plasma halflife
of timolol is approximately 5.5 hours.The extensive
tissue distribution of timolol into lung, liver, and kidney
is similar to that of other -blockers. Approximately
70% of the drug is excreted in the urine within 24 hours,
mostly as highly polar unconjugated metabolites. Only
6% of an administered dose is recovered in the feces.
Although timolol is approved for the topical treatment
of elevated intraocular pressure, there is limited information
about its pharmacokinetics following administration
by this route.The drug apparently can reach the
systemic circulation after intraocular instillation, but
plasma levels are only about 7% of those achieved in
the aqueous humor.