Synthesis
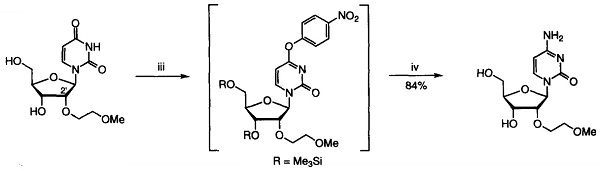
2'-O-(2-Methoxyethyl)uridine could be converted into the corresponding cytidine derivative (2'-O-(2-Methoxyethyl)cytidine) by the 4-nitrophenylation[1]. 2'-O-(2-Methoxyethyl)uridine, l-methylpyrrolidine, chlorotrimethylsilane and dry acetonitrile were stirred together at rt. After 1 h, the reaction mixture was cooled to 0 ℃, and trifluoroacetic anhydride was added dropwise over 5 min. After a further period of 30 rain at 0 ℃, 4-nitrophenol was added to the stirred reactants which were maintained at 0 °C. After 3 h, the products were poured into saturated aqueous sodium hydrogen carbonate, and the resulting mixture was extracted with dichloromethane. The combined organic layers were dried, and evaporated under reduced pressure. Concentrated aqueous ammonia was added to a stirred solution of the residue in dioxane, contained in a sealed flask that was then heated at 55°C for 24 h. The resulting yellow solution was concentrated under reduced pressure and the residue was evaporated with absolute ethanol. The products were fractionated by short column chromatography on silica gel and were evaporated under reduced pressure to give 2'-O-(2-Methoxyethyl)cytidine as an off-white solid.