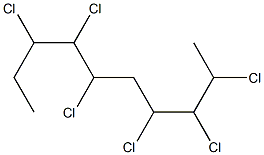
Chemical Properties
Chlorinated paraffins are chlorinated long-chain aliphatic compounds. They exist as light-yellow to amber-colored viscous, oily liquids that are usually odorless. The commercial products are complex mixtures that contain paraffins with various carbon-chain lengths and varying chlorine content. The commercial products normally contain stabilizers to inhibit decomposition and may contain isoparaffins (< 1%), aromatic compounds (< 0.1%), and metals as contaminants. Chlorinated paraffins are practically insoluble in water, but many products may be emulsified with water. They are miscible with benzene, chloroform, ether, and carbon tetrachloride, slightly soluble in alcohol, and soluble in most aromatic, aliphatic, and terpene hydrocarbons, ketones, esters, and vegetable and animal oils. Chlorinated paraffins have low volatility and are nonflammable. When heated to decomposition, they emit toxic fumes of hydrochloric acid and other chlorinated compounds. The physical and chemical properties of these chemical mixtures are variable. The octanol-water partition coefficient (log K
ow) ranges from 4.48 to 7.38 (IPCS 1996, HSDB 2009).
Uses
Chlorinated paraffins are used as extreme-pressure-lubricant additives in metalworking fluids; as flame retardants in plastics, rubber, and paints; to improve water resistance of paints and fabrics; and as a secondary plasticizer in polyvinyl chloride. Small amounts are also used in caulks, sealants, adhesives, detergents, inks, finished leather, and other miscellaneous products, and are allowed as an indirect food additive (NTP 1986, CMR 2002, HSDB 2009, FDA 2010). In the United States, about 50% of chlorinated paraffins are used in metalworking fluids, 20% in plastics additives, 12% in rubber, 9% in coatings, 6% in adhesives, caulks, and sealants, and the remaining 3% for miscellaneous purposes (CMR 2002). Chlorinated paraffins have replaced polychlorinated biphenyls as fire-retardant lubricants (NTP 1986). Between 1914 and 1918, large amounts of chlorinated paraffins were used as solvents for dichloramine-T in antiseptic nasal and throat sprays (IPCS 1996).
General Description
Clear colorless to light amber viscous liquid. Slight odor or no odor.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
CHLOROPARAFFIN gives evidence of instability at temperatures greater than 77° F. CHLOROPARAFFIN discolors in sunlight. CHLOROPARAFFIN is sensitive to prolonged exposure to heat or light (can darken). Aluminum, zinc or iron will catalyze this decomposition. CHLOROPARAFFIN is incompatible with strong oxidizing and reducing agents. CHLOROPARAFFIN is also incompatible with strong alkalis.
Fire Hazard
CHLOROPARAFFIN is nonflammable.
Safety Profile
Confirmed carcinogen with carcinogenic and neoplastigenic data. When heated to decomposition it emits acrid smoke and irritating fumes.
Carcinogenicity
Chlorinated paraffins (C12, 60% chlorine) are reasonably anticipated to be human carcinogens based on sufficient evidence of carcinogenicity from studies in experimental animals.