Description
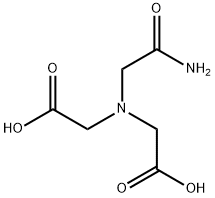
N-(2-Acetamido)iminodiacetic acid is a zwitterionic buffer used in biochemistry and molecular biology. It is one of the Good buffers developed in the 1960′s to provide buffers in the pH range of 6.15 - 8.35 for wide applicability to biochemical studies. The pioneering publication by Good and co-workers describes the synthesis of ADA and its physical properties. The useful range of ADA buffer in aqueous solution is 6.0 - 7.2.
Chemical Properties
N-(2-Acetamido)iminodiacetic acid is white to almost white powder
Uses
N-(2-Acetamido)iminodiacetic acid (ADA) could prevents oxidation and irreversible denaturation of proteins while conducting gel electrophoresis. Furthermore studies suggest that ADA interferes with BCA and Lowry protein assays. Also ADA often chelates metal ions such as Mn(II), Cu(II), Ni(II), Zn(II), and Co(II) in a 2:1 at or below physiological pH values. Moreover, ADA is soluble in sodium hydroxide and absorbs UV radiation in the range below 260nm.
Uses
N-(2-Acetamido)iminodiacetic acid is buffer for biological systems and chelator for metals.
Uses
Biological buffer component with sodium hydroxide and sodium chloride (pH 5.67-7.57, useful pH range 6.4-7.4).Used to prepare immobilized pH gradients.
Application
ADA, or N-(2-Acetamido)iminodiacetic acid, can be used to study biological buffers and zwitterionic compounds. ADA has been used in a study to describe the application of scanning capacitively coupled contactless conductivity detection (SC(4)D) for the determination of pH dependant behaviour of two aminopolycarboxylates immobilised onto the surface of a monolithic capillary column.
Biological buffer component with sodium hydroxide and sodium chloride (pH 5.67-7.57, useful pH range 6.4-7.4). Used to prepare immobilized pH gradients.
Definition
ChEBI: 2,2'-[(2-amino-2-oxoethyl)imino]diacetic acid is a tricarboxylic acid amide that is a Good's buffer substance, pKa = 6.6 at 20 ℃. It is a dicarboxylic acid, a tricarboxylic acid amide and an ADA. It is functionally related to a nitrilotriacetic acid. It is a conjugate acid of a 2,2'-[(2-amino-2-oxoethyl)imino]diacetate(1-).
Flammability and Explosibility
Not classified
Purification Methods
Dissolve ADA in water, add one equivalent of NaOH solution (to final pH of 8-9), then acidify with HCl to precipitate the free acid. This is filtered off, washed with water and dried in vacuo. [Beilstein 4 IV 2441.]