Uses
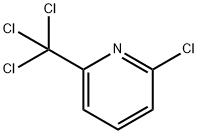
2-Chloro-6-(trichloromethyl)pyridine is a nitrification inhibitor used to limit NO and N2O emissions from crops. Improves nitrogen use efficiency. Environmental toxin on US EPA Toxic Release Inventory list (TRI) list.
Chemical Properties
Crystals.
Physical properties
Colorless to white crystalline solid with a mild, sweet odor
Uses
2-Chloro-6-(trichloromethyl)pyridine is
Uses
Bactericide used to inhibit Nitrosomonas spp. from oxidizing ammonium ions in soil.
Uses
Fertilizer additive to control nitrification and prevent loss of soil nitrogen.
Definition
ChEBI: Nitrapyrin is a chloropyridine that is 2-chloropyridine which is substituted by a trichloromethyl group at position 6. It is a nitrification inhibitor that is co-applied with nitrogen fertilizer in agroecosystems. It has a role as a nitrification inhibitor, an antibacterial agent and an agrochemical.
General Description
Colorless crystals or off-white crystalline solid.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Nitrapyrin is a base. Reacts exothermically with acids.
Hazard
Liver damage. Questionable carcinogen.
Fire Hazard
Flash point data for Nitrapyrin are not available. Nitrapyrin is probably combustible.
Flammability and Explosibility
Non flammable
Agricultural Uses
Nitrapyrin, also called N serve, is a nitrification
inhibitor
Safety Profile
Poison by ingestion.
Moderately toxic by skin contact.
Experimental reproductive effects. Mutation
data reported. When heated to
decomposition it emits very toxic fumes of
Cland NOx.
Environmental Fate
Biological. 6-Chloropicolinic acid and carbon dioxide were reported as biodegradation
products (Verschueren, 1983).
Soil. Hydrolyzes in soil to 6-chloropyridine-2-carboxylic acid (Worthing and Hance,
1991).
Photolytic. Photolysis of nitrapyrin in water yielded 6-chloropicolinic acid, 6-hydroxypicolinic
acid and an unidentified polar material (Verschueren, 1983).
Chemical/Physical. Emits toxic fumes of nitrogen oxides and chlorides when heated
to decomposition (Sax and Lewis, 1987; Lewis, 1990).