Synthesis
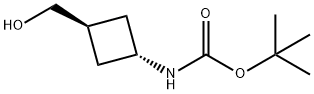
To a stirred solution of methyl (1R,3R)-3-((tert-butoxycarbonyl)amino)cyclobutane-1-carboxylate (1.0 g, 4.4 mmol) in THF (20 mL) was added 3M lithium borohydride (9 mL, 9 mmol) at 0° C. Then the reaction mixture was heated and stirred at 60° C for 3 h. The reaction mixture was then transferred into dilute NaOH and the resulting mixture was extracted using ethyl acetate (3×50 mL). The combined organic layer was dried over anhydrous sodium sulfate and filtered. The filtrate was evaporated under reduced pressure and the crude product was purified using silica gel column chromatography (3% MeOH-DCM) to give tert-Butyl (trans-3-(hydroxymethyl)cyclobutyl)carbamate as a white solid (0.8 g, 91%).