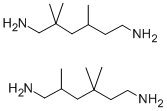
General Description
A colorless to light yellow liquid. Insoluble in water and less dense than water. Contact may severely irritate skin, eyes and mucous membranes. May be toxic by ingestion, inhalation and skin absorption. Used to make other chemicals.
Reactivity Profile
Amines, such as TRIMETHYLHEXAMETHYLENEDIAMINE, are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides.