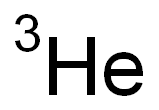Chemical Properties
Helium-3,3He, has been known as a stable isotope since the middle-1930s and it was suspected that its properties were markedly different from the common isotope, helium-4.
History
The development of nuclear fusion devices in the 1950s yielded workable quantities of pure helium-3 as a decay product from the large tritium inventory implicit in maintaining an arsenal of fusion weapons.
Definition
ChEBI: The stable isotope of helium with relative atomic mass 3.016029. The least abundant (0.000137 atom percent) isotope of naturally occurring helium.