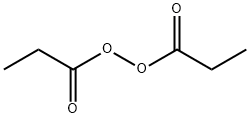
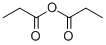
Uses
Initiator in polymerization reactions, such as
the high-pressure polymerization of ethylene.
Definition
Avail-
able as a 25% solution in a high-boiling hydrocar-
bon, flash p 125F (51.6C).
General Description
Available only as a 25% solution. Pure material poses a severe explosion hazard. Used as an initiator in polymerization reactions.
Air & Water Reactions
Highly flammable. May ignite spontaneously if exposed to air.
Reactivity Profile
Dipropionyl peroxide(in solution,content≤27%) is a good oxidizing agent. May cause ignition of organic compounds on contact . Reacts violently with strongly reduced material such as sulfides, nitrides, and hydrides. Many produce explosions or generate gases (toxic and nontoxic). Generally, dilute solutions (<70%) are safe, but the presence of a catalyst (often a transition metal such as cobalt, iron, manganese, nickel, or vanadium) as an impurity may even then cause rapid decomposition, a buildup of heat, and even an explosion. May become explosive when evaporated to dryness or near-dryness. May explode from heat, contamination or loss of temperature control.
Hazard
Strong oxidizing agent, may explode if
shocked or heated.
Safety Profile
material explodes at
room temperature. When heated to
decomposition it emits acrid smoke and
fumes. See also PEROXIDES.