Synthesis
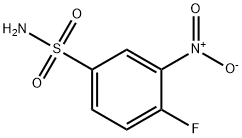
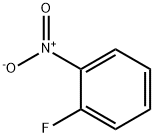
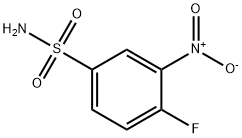
General procedure for the synthesis of 4-fluoro-3-nitrobenzenesulfonamide from 1-fluoro-2-nitrobenzene: 1-fluoro-2-nitrobenzene (4.0 g, 28.4 mmol) was dissolved in chlorosulfonic acid (25 mL), and the reaction was stirred at 120°C overnight. Upon completion of the reaction, it was cooled to room temperature and quenched by slow pouring into ice water. The reaction mixture was extracted with ethyl acetate (50 mL x 3), the organic layers were combined and concentrated under reduced pressure to remove the solvent. The crude product was redissolved in isopropanol and cooled to -60°C. At this temperature, ammonium hydroxide solution was added dropwise and stirring was continued for 1 hour. Subsequently, 6M hydrochloric acid (8 mL) was added to neutralize the reaction and the reaction mixture was warmed to room temperature and concentrated to dryness. The intermediate 4-fluoro-3-nitrobenzenesulfonamide (5.1 g, 82% yield) was finally obtained as a white solid.1H NMR (400 MHz, DMSO-d6) δ: 8.52 (dd, 1H), 8.20 (dq, 1H), 7.84 (dt, 1H), 7.73 (s, 2H).
References
[1] Patent: WO2017/123616, 2017, A1. Location in patent: Paragraph 00160
[2] Bioorganic and Medicinal Chemistry Letters, 2016, vol. 26, # 21, p. 5207 - 5211
[3] Chemistry - A European Journal, 2018, vol. 24, # 52, p. 13762 - 13766
[4] Journal of Medicinal Chemistry, 2006, vol. 49, # 3, p. 1165 - 1181
[5] Patent: US2011/237553, 2011, A1. Location in patent: Page/Page column 19