Pharmacology and mechanism of action
Oxamniquine is a tetrahydroquinoline derivative effective in the treatment of Schistosoma(s) mansoni infections. The male worms are more susceptible to the drug effects than the female ones. It has no therapeutic value against other Schistosoma species. In experimental animal models, the drug causes a shift of the worms from the mesenteric veins to the liver where the male and the female decouple. The male worms preferentially concentrate the drug and die in the liver. The unpaired females return to the mesenteric vessels where they cease laying eggs and eventually die
[1]. The mechanism of action of Oxamniquine is unknown. The drug may induce its action by inhibiting DNA synthesis. When the drug was administered to rats infected with S. mansoni, it inhibited the synthesis of macromolecules in sensitive parasites and not in the resistant ones
[2].
Indications
Oxamniquine is used against S. mansoni infections, including advanced cases with hepatomegaly, ascites or with colonic polyposis.
Side effects
Oxamniquine is generally well tolerated even during large scale treatment programmes. The only significant common side effect reported is mild to moderate dizziness with or without drowsiness, reported by up to 40% of treated patients. It starts up to 3 hours after a dose and usually lasts for 3 to 6 hours. Other side effects include nausea, vomiting, abdominal pain, and diarrhoea
[3]. Transient fever, 38 to 39°C, peripheral blood eosinophilia and pulmonary infiltrates (Loeffler’s syndrome) have been reported mainly from Egyptian patients 24 to 72 hours after completing a 3-day course of therapy
[4]. The cause seems to be unknown. A number of reports of epileptiform convulsions have been reported in patients suspected with
[5] or without
[6,7]a history of epilepsy. More severe neuropsychiatric symptoms such as severe headache, hallucinations, episodes of fainting, severe amnesia, total disorientation in space and time and confusion have been rarely reported
[8,9].
Discoloration of the urine from orange to red may follow after the drug treatment (most likely due to a metabolite)
[10]. This is transitory and harmless, nevertheless patients should be informed about it.
Contraindications and precautions
Patients with pre-existing central nervous system disturbances such as epilepsy or psychiatric disorders should be treated with caution.
Preparations
• Vansil® (Pfizer). Capsules 250 mg. Oral suspension 50 mg/ml.
• Mansil® (Pfizer). Capsules 250 mg. Oral suspension 50 mg/ml.
References
1. Webster LT Jr (1990). Drugs used in the chemotherapy of helminthiasis. In: Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 8th edn, edited by A.G.Gilman, T.W.Rall, A.S.Nies, P Taylor, (New York: Pergamon Press), pp. 966.
2. Pica-Mattoccia L, Coli D (1985). Studies on the mode of action of oxamniquine and related schistosomicidal drugs. Am J Trop Med Hyg, 34, 112–118.
3. Foster R (1987). A review of clinical experience with oxamniquine. Trans R Soc Trop Med Hyg, 81, 55–59.
4. Higashi GI, Farid Z (1979). Oxamniquine fever: drug-induced or immune-complex reaction BMJ, ii, 830.
5. Krajden S, Keystone JS, Glenn C (1983). Safety and toxicity of oxamniquine in the treatment of Schistosoma mansoni infections, with particular reference to electroencephalographic abnormalities. Amer J Trop Med Hyg, 32, 1344–1346.
6. Stockvis H, Bauer AGC, Stuiver PC, Malcolm AD, Overbosche D (1986). Seizures associated with oxamniquine therapy. Am J Trop Med Hyg, 35, 330–331.
7. Al-aska AK (1985). Treatment of Schistosoma mansoni infection with oxamniquine in Riyadh, Saudi Arabia. Trop Med Parasitol, 36, 213–214.
8. Katz N, Grinbaum E, Chaves A, Zicker F, Pellegrind J (1976). Clinical trials with oxamniquine by oral route, in Schistosoma mansoni. Rev Inst Med Trop São Paulo, 18, 371–377.
9. Chunge CN, Kimani RG, Gachihi G, Mkoji G, Kamau T, Rashid JR (1985). Serious side effects
10. Anthelminthics. Martindale: The Extra Pharmacopoeia, 30th edn (1993), (London: Pharmaceutical Press), pp: 4950.
Description
Oxamniquine was originally investigated in the 1960s and was found to have limited antiprotozoal
activity, with activity against Schi stosoma mansoni but no activity against the other two
schistosomal organisms. In addition, the drug is stage specific, with activity against cercariae and
very young schistosomula and adult worms. For reasons that remain unknown, the drug is more
effective against adult male worms than against female worms. The drug has structural similarity to
hycanthone, which is no longer used because of severe toxicity and teratogenic effects.
Originator
Vansil, Pfizer , US ,1980
Definition
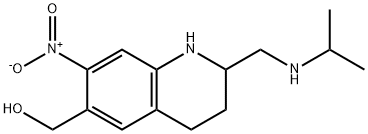
ChEBI: A member of the class of quinolines that is 1,2,3,4-tetrahydroquinoline which is substituted at positions 2, 6, and 7 by (isopropylamino)methyl, hydroxymethyl, and nitro groups, respectively.
Indications
Oxamniquine (Vansil) is a tetrahydroquinoline that
stimulates parasite muscular activity at low concentrations
but causes paralysis at higher concentrations. The
drug may act by esterification and binding of DNA,
leading to the death of the schistosome by interruption
of its nucleic acid and protein synthesis. The fluke may
esterify oxamniquine to produce a reactive metabolite
that alkylates parasite DNA. Resistance results from
absent or defective esterifying activity of the drug.
Oxamniquine has a restricted range of efficacy, being
active only against S. mansoni infections.
Oxamniquine is given orally and is readily absorbed
from the intestinal tract. Peak concentrations in plasma
are obtained in about 3 hours. The drug is excreted in
urine mostly as a 6-carboxyl derivative.
Side effects include CNS toxicity with unsteadiness
and occasionally seizures, especially in patients with a
history of seizures. It is contraindicated in pregnancy.
Manufacturing Process
(1) Four fermenters are set up, each one of which contained 2.0 liters of the
following medium, sterilized for 35 minutes at 15 psi, respectively
The fermenters are inoculated with 7.5% by volume of a 24-hour old culture of Aspergillus sclerotiorum Huber grown at 28°C in 50 ml aliquots of the above described soybean-glucose medium contained in 300 ml Erlenmeyer flasks, placed on a shaker rotating at approximately 230 rpm. The inoculated fermenters are agitated at 1,380 rpm and each aerated with 1 liter of air per minute and at a temperature of 28°C for 47 hours. A silicone antifoam is added when required. At the end of the 47 hour period, the pH of the fermentation broth rose to 6.8 to 6.9. Sulfuric acid is then added with sterile precautions to restore the pH to 6.5.
(2) 0.75 g of 2-isopropylaminomethyl-6-methyl-7-nitro-1,2,3,4tetrahydroquinoline as hydrogen maleate, dissolved in 75 ml of sterile water, is added to each of the four fermenters and agitation and aeration are continued for a further 23 hours. The whole fermentation broths from each fermenter are pooled, the pH adjusted to 8.0 with sodium hydroxide and the 8.2 liters of fermentation broth thus obtained are extracted by agitating vigorously with 16.4 liters of methylene chloride for 10 minutes. The solvent extract is then dried over anhydrous sodium sulfate and subsequently evaporated to dryness at a temperature below 40°C (dry weight 5.567 g).
(3) The dark brown residue from (2) is extracted four times with methanol at room temperature, decanting the solution from the insoluble material. The combined methanol extracts, total volume about 200 ml, are then filtered and treated with 3 g of sodium borohydride, added in portions over a period of 30 minutes with stirring, to reduce any 6-formyl compound present to the 6hydroxymethyl compound. The methanol solution is then allowed to stand overnight at room temperature and is thereafter diluted with 1 liter of ether. The solution is washed 4 times with 500 ml of water and the resulting pale yellow ethereal solution is dried over magnesium sulfate. The ether is next removed by vacuum distillation from a water bath at 40°C. The residue is dissolved in about 75 ml of isopropanol at 50°C, filtered to remove any insoluble particles and cooled overnight in the refrigerator. The product is collected and dried in vacuo to yield 0.5 g of 6-hydroxymethyl-2isopropylaminomethyl-7-nitro-1,2,3,4-tetrahydroquinoline as pale yellow crystals of melting point 147°C to 149°C. A further 0.5 g of crude material is obtained from the mother liquors of the recrystallization. Total yield is therefore 1.0 g (0.0036 mol) from 3.0 g (0.0079 mol) of starting material, i.e., 45% of the theoretical amount.
brand name
Vansil (Pfizer).
Therapeutic Function
Antischistosomal
Antimicrobial activity
Activity is restricted to
Schistosoma mansoni. Some strains, particularly those in Egypt and Southern Africa, require higher doses for efficacy owing to innate tolerance.
Pharmaceutical Applications
A synthetic quinolinemethanol, available for oral administration.
Mechanism of action
Oxamniquine is activated via esterification to a biological ester that spontaneously dissociates to
an electrophile, which alkylates the helminth DNA, leading to irreversible inhibition of nucleic acid
metabolism. Resistant helminths do not esterify oxamniquine; therefore, activation
does not occur. Other metabolic reactions consist of oxidative reactions, leading to inactivation. The metabolites are excreted primarily in the urine.
Pharmacokinetics
It is rapidly absorbed after oral administration, achieving a
peak concentration of 0.3–2.5 mg/L 1–3 h after an oral dose
of 15 mg/kg body weight. Peak levels following intramuscular
treatment at 7.5 mg/kg generally do not exceed 0.15 mg/L. It
is extensively metabolized to biologically inactive 6-carboxylic
and 2-carboxylic acid derivatives, which are excreted in the
urine, mostly within 12 h.
Clinical Use
1,2,3,4-Tetrahydro-2-[(isopropylamino)methyl]-7-nitro-6-quinolinemethanol (Vansil) is an antischistosomal agent thatis indicated for the treatment of Schistosoma mansoni (intestinalschistosomiasis) infection. It has been shown to inhibitDNA, RNA, and protein synthesis in schistosomes. The6-hydroxymethyl group is critical for activity; metabolic activationof precursor 6-methyl derivatives is critical. Theoral bioavailability of oxamniquine is good; effectiveplasma levels are achieved in 1 to 1.5 hours. The plasmahalf-life is 1 to 2.5 hours. The drug is extensively metabolizedto inactive metabolites, of which the principal one isthe 6-carboxy derivative.
The free base occurs as a yellow crystalline solid thatis slightly soluble in water but soluble in dilute aqueousmineral acids and soluble in most organic solvents. Itis available in capsules containing 250 mg of the drug.Oxamniquine is generally well tolerated. Dizziness anddrowsiness are common, but transitory, side effects. Seriousreactions, such as epileptiform convulsions, are rare.
Clinical Use
Infection with S. mansoni
Side effects
Dizziness, sleepiness, nausea and headache occur frequently.
Other side effects are probably due to the death and disintegration
of the worms in the liver. Following treatment, urine
may become red.
Safety Profile
Poison by ingestion, intraperitoneal and intramuscular routes. Human mutation data reported. An antischistosomal agent. When heated to decomposition it emits toxic fumes of NOx.