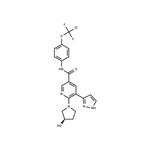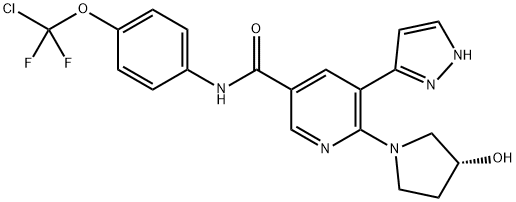
(R)-N- (4-(chlorodifluoromethoxy)phenyl)- 6-(3- hydroxypyrrolidin-1- yl)-5- (1H-pyrazol- 5-yl)nicotinamide
- Product Name(R)-N- (4-(chlorodifluoromethoxy)phenyl)- 6-(3- hydroxypyrrolidin-1- yl)-5- (1H-pyrazol- 5-yl)nicotinamide
- CAS1492952-76-7
- CBNumberCB23133462
- MFC20H18ClF2N5O3
- MW449.84
- MDL NumberMFCD31560488
- MOL File1492952-76-7.mol
Chemical Properties
| Boiling point | 631.7±55.0 °C(Predicted) |
| Density | 1.518±0.06 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | DMSO:93.0(Max Conc. mg/mL);206.74(Max Conc. mM) Ethanol:90.0(Max Conc. mg/mL);200.07(Max Conc. mM) |
| form | A crystalline solid |
| pka | 10.81±0.70(Predicted) |
| color | White to off-white |
| FDA UNII | L1F3R18W77 |
| NCI Drug Dictionary | asciminib |
Safety
| Symbol(GHS) |

|
|||||||||
| Signal word | Warning | |||||||||
| Hazard statements | H302-H315-H319-H335 | |||||||||
| Precautionary statements | P261-P305+P351+P338 | |||||||||
| NFPA 704: |
|