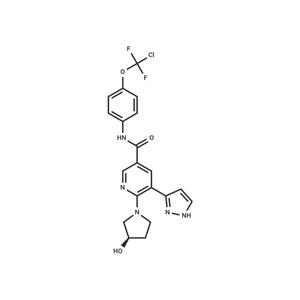
| Name | Asciminib |
| Description | Asciminib (ABL001) (ABL001) is a potent and selective Bcr-Abl inhibitor (Kd: 0.5–0.8nM). |
| Cell Research | Cells were resuspended in fresh culture media before culture in 24-well plates in the presence of TKI or asciminib at a density of 2 × 105 cells/mL. Plates were seeded with 1 mL of cell suspension and incubated for 72 h before cell viability determination with 7-aminoactinomycin (7-AAD) and Phycoerythrin (PE)-conjugated Annexin V. Flow cytometric analysis was conducted with a BD LSRFortessa X-20 and FACSDiva software. The lethal dose of asciminib (LD50 asciminib), imatinib (LD50 IM), nilotinib (LD50 NIL) and dasatinib (LD50 DAS) required to cause 50% death of cells was calculated [2]. |
| Animal Research | Asciminib efficacy in three patient-derived ALL systemic xenograft models (ALL-7015, AL-7119 and AL-7155) is assessed by FACS monitoring of the percentage of CD45+ cells per live cell in blood samples taken at varying time points after dosing with either 7.5 mg/kg BID (group 2) or 30 mg/kg BID (group 3) asciminib for 3 weeks [1]. |
| In vitro | In Asciminib-transformed Ba/F3 cells grown without IL-3, ABL001 had an anti-proliferative IC50 value of 0.25nM. By contrast, the addition of IL-3 to bypass Asciminib dependence renders these cells insensitive to ABL001. In the CML blast-phase cell line KCL-22, ABL001 inhibited phosphorylation of both STAT5 (Tyr694; pSTAT5) and Asciminib (Tyr245; pAsciminib) after 1h using concentrations that correlate with those required for inhibition of cell proliferation [1]. K562-Dox and K562-ABCG2 cells demonstrated increased LD50 (asciminib) vs K562 control cells: 256 and 299 nM respectively vs 24 nM. Sensitivity was completely restored with specific inhibitors cyclosporine (ABCB1) and Ko143 (ABCG2): K562-Dox LD50 (asciminib+cyclosporine) = 13 nM, K562-ABCG2 LD50 (asciminib+Ko143) = 15 nM [2]. |
| In vivo | Single doses of 7.5, 15 and 30mg kg1 ABL001, administered to mice bearing KCL22 xenografts, inhibited pSTAT5 (Tyr694), which returned to baseline at 10, 12 and 16–20h after administration of the dose, respectively. In mice implanted with KCL-22 tumours, the minimum dose of ABL001 required for complete regression was 7.5mg/kg twice a day (BID) or 30mg/kg once a day (QD), and was tolerated at doses up to 250mg kg1 BID. Similarly, in xenografts derived from patients with Ph+ ALL, treatment with 7.5 and 30mg/kg ABL001 led to regressions that were maintained during dosing [1]. |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. |
| Solubility Information | H2O : Insoluble
DMSO : 55 mg/mL (122.27 mM)
|
| Keywords | Bcr-Abl | ABL-001 | inhibit | ABL 001 | Inhibitor | Asciminib |
| Inhibitors Related | Imatinib Mesylate | Bosutinib hydrate | Pivanex | Dasatinib | GNF-5 | Ponatinib | Nilotinib monohydrochloride monohydrate | KW-2449 | Nilotinib | Dasatinib monohydrate | Imatinib | Masitinib |
| Related Compound Libraries | Bioactive Compound Library | Tyrosine Kinase Inhibitor Library | EMA Approved Drug Library | Anti-Cancer Clinical Compound Library | Inhibitor Library | FDA-Approved Drug Library | FDA-Approved Kinase Inhibitor Library | Anti-Cancer Approved Drug Library | Bioactive Compounds Library Max | Anti-Cancer Active Compound Library |

 United States
United States