Synthesis
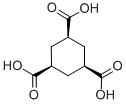
A mixture of 18 g (85 mmol) of trimesic acid, 2 g (10% mass equiv) of 5% rhodium on alumina,22 and 300 mL of water was hydrogenated at 70 °C and 50 psi. Uptake was complete in 7 h and the yield was almost quantitative. The filtrate was concentrated to give a white solid. The acid obtained was mostly the cis,cis form (cis,cis/cis,trans = 7:1). Recrystalization from ethanol-toluene solution gave the cis,cis isomer, 1,3,5-Cyclohexanetricarboxylic acid, quantitative yield mp 218-219 °C (lit.23 mp 218 °C); 1H NMR (500 MHz, CDCl3) δ11.51 (br s, 3H), 1.91 (dd, J = 5.5 Hz, J = 5.0 Hz, 3H), 1.83 (dd, J = 13.0 Hz, J = 5.0 Hz, 3H), 1.02 (dd, J = 13.0 Hz, J = 5.5 Hz, 3H); 13C NMR (500 MHz, CDCl3) δ175.44, 40.79, 29.91; IR (cm-1) 2917, 2850, 1708, 1616, 1418, 1289, 1261.

Purification Methods
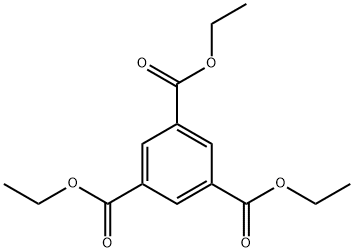
Purify it also by conversion to the triethyl ester b 217-218o/10mm, 151o/1mm, the distillate solidifies on cooling, m 36-37o, which is hydrolysed by boiling in aqueous HCl. The trimethyl ester can be distilled and recrystallised from Et2O, m 48-49o. [Newman & Lawrie J Am Chem Soc 76 4598 1954, Lukes &S Galik Coll Czech Chem Comm 19 712 1954, Beilstein 9 III 4749.]