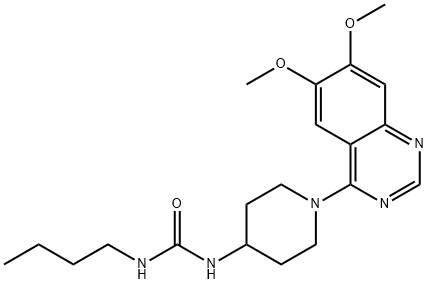
Manufacturing Process
4-Chloro-6,7-dimethoxyquinazoline (45.0 g), 4-(3-n-butylureido)piperidine
monhydrochloride (80.0 g) and triethylamine (140 ml) were refluxed in
ethanol (450 ml) for 1.25 h. The mixture was then concentrated in vacuo and
the resultant solid was stirred in water which was then basified to pH 11 with
5 N NaOH solution. The suspension was shaken with chloroform and the
organic layer was separated, dried (Na2CO3) and evaporated to dryness in
vacuo to give a yellow oily solid. Trituration with ether followed by
recrystallization from ethanol gave the product (37.0 g) with small traces of
impurities, wich were removed by running a chloroform solution of it down a
glass column packed with "Florisil" and eluting with 10% isopropanol in
chloroform.
After evaporation, appropriate fractions were bulked to give a pure 4-(4-[3-nbutylureido]
piperidino)-6,7-dimethoxyquinazoline, melting point 204-205°C
(21.0 g; recrystallized from ethanol).