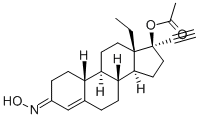
Description
Norgestimate is an orally-effective
progestogen, recently launched in combination with ethinyl estradiol as an oral contraceptive.
Chemical Properties
White Crystalline Solid
Uses
Progestogen. In combination with estrogen as oral contraceptive.
Definition
ChEBI: Norgestimate is a steroid ester, a ketoxime and a terminal acetylenic compound. It has a role as a contraceptive drug, a progestin and a synthetic oral contraceptive.
Manufacturing Process
A solution of 4.5 g of D-17β-acetoxy-13β-ethyl-17α-ethynyl-gon-4-en-3-one in 15 ml of pyridine and 2.0 g of hydroxylamine hydrochloride hydroxylamine hydrochloride is heated on a steam bath for 45 min. It is then cooled and poured into a large amount of ice-water, after which the solid which is thus produced is filtered off and air dried. Recrystallization from methylene chloride-ethanol gives D-17β-acetoxy-13α-ethyl-17α-ethynyl-gon-4-en-one oxime, m.p. 214-218°C; [α]D25 = +41°.
Therapeutic Function
Progestin
General Description
Norgestimate, (17α)-17-acetyloxy-13-ethyl-18,19-dinor-pregn-4-en-20yn-3-one oxime, is a 19-nortestosterone, 3-oxime prodrug that is orally active andused with an estrogen in oral contraceptive products. It hasminimal androgenic action. Norgestimate is metabolized to17-deacetylnorgestimate (norelgestromin) and norgestrel,which provide the progestational action.
in vitro
norgestimate was found that, unlike other 19-nortestosterone derivatives, showed high selectivity for the progesterone receptor and low androgenic activity. moreover, norgestimate and its main active metabolite norelgestromin could not bind to or occupy sex hormone-binding globulin [1].
in vivo
the androgenic and the progestational activity of norgestimate were compared in two animal studies. it was found the difference in the pharmacological response in norgestimate treated rats was equivalent to the difference in the exposure of the animals to either directly administered or metabolically derived levonorgestrel [2].
Metabolism
Norgestimate is considered to be a pro-proges tin (prodrug), because it rapidly undergoes a two-s tep m etabolic transformation to form two active products, norelgestromine (levonorgestrel 3-oxime) and levonorgestrel. Deacetylation occurs in the intestine and liver, whereas convers ion of the 3-oxime to the corresponding ketone occurs primarily in the liver. Unlike the other progestins mentioned, norgestimate and its metabolites are not bound to SHBG.
References
[1] thomas l. lemke; david a. williams (2008). foye's principles of medicinal chemistry. lippincott williams & wilkins. pp. 1316–. isbn 978-0-7817-6879-5.
[2] kuhnz w, beier s. comparative progestational and androgenic activity of norgestimate and levonorgestrel in the rat. contraception. 1994 mar;49(3):275-89.
[3] https://en. wikipedia.org/wiki/norgestimate