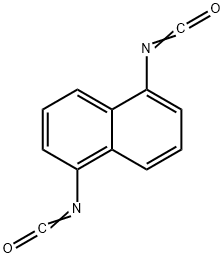
Description
Naphthalene diisocyanate (NDI) occurs as white to lightyellow
crystalline flakes with a characteristic odor. NDI is
incompatible with many classes of compounds, reacting
exothermically to release toxic gases. Reactions with amines,
aldehydes, alcohols, alkali metals, ketones, mercaptans, strong
oxidizers, hydrides, phenols, and peroxides can cause vigorous
releases of heat. Acids and bases initiate polymerization reactions.
NDI can react with water to form amines and liberate
carbon dioxide.
Chemical Properties
Naphthylene 1,5-diisocyanate is a solid, m.p. 128°C. It has a lower vapour
pressure than tolylene diisocyanate and is therefore less toxic in use; it does,
however, have sensitizing properties.
Uses
Naphthylene 1,5-diisocyanate is mainly
used for the production of elastomers.
Uses
NDI is used as a curing agent in the manufacture of elastomers.
Uses
Manufacture of polyurethane solid elastomers.
Definition
ChEBI: 1,5-Naphthalene diisocyanate is a member of naphthalenes.
Preparation
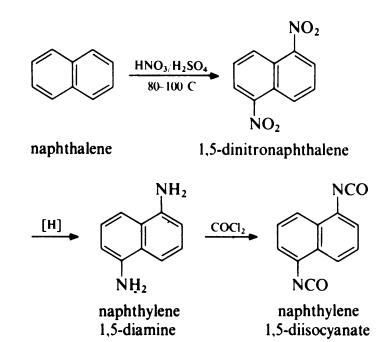
Naphthylene 1,5-diisocyanate (NDI) is prepared from naphthalene as follows:
General Description
White to light-yellow crystalline flakes.
Reactivity Profile
Isocyanates and thioisocyanates, such as 1,5-Naphthalene diisocyanate, are incompatible with many classes of compounds, reacting exothermically to release toxic gases. Reactions with amines, aldehydes, alcohols, alkali metals, ketones, mercaptans, strong oxidizers, hydrides, phenols, and peroxides can cause vigorous releases of heat. Acids and bases initiate polymerization reactions in these materials. Some isocyanates react with water to form amines and liberate carbon dioxide. Base-catalysed reactions of isocyanates with alcohols should be carried out in inert solvents. Such reactions in the absence of solvents often occur with explosive violence, [Wischmeyer(1969)].
Hazard
Irritant. Questionable carcinogen.
Flammability and Explosibility
Non flammable
Safety Profile
A powerful allergen. An
irritant. Questionable carcinogen. When
heated to decomposition it emits toxic
fumes of NOx.
Environmental Fate
NDI is a synthetic organic chemical. It is a natural derivative of
primary amines with the general formula R–N]C]O which
does not occur naturally in the environment. At room temperature
it can be a liquid or crystal. It is miscible with alcohol,
diglycol, monoethyl ether, ether, acetone, carbon tetrachloride,
benzene, chlorobenzene, kerosene, and olive oil; however, it
may react violently with alcohol, water, acid, bases, and strong
alkaline materials and tertiary amines and generate enough heat
to self-ignite and release toxic combustion products. NDI is not
readily biodegradable; however, it reacts with water and most
acids producing unstable carbonic acids, which subsequently
decarboxylate yielding relatively chemically inert and insoluble
polymeric urea. While these polyureas are persistent, studies
have indicated that they pose virtually no potential for adverse
impacts on the aquatic environment. Due to hydrolysis in water,
bioaccumulation of NDI is not expected. Since the hydrolysis
products formed are irritants, there is a potential for inhalation
exposure. The degree stability is a function of humidity.
Toxicity evaluation
The toxicological properties of isocyanates are attributed to
the –N=C=O group. It is thought to react vigorously and
exothermically with water forming an unstable carbamic
acid that dissociates to form a primary amine with liberation
of CO2. Hence, the primary amine will react further
generating a urea derivative. Isocyanates also react readily
with all organic compounds resulting in polymerization.
Such reactions denature proteins, form abnormal crosslinkages,
and generally disorganize the protein resulting in
alteration of its normal function. This reactivity with
proteins can account for its potency as a sensitizing agent.
An IgE- or IgG-mediated mechanism has been proposed,
but has not been definitively linked to isocyanate exposure.
There is also evidence that inflammation and morphological
changes of the bronchia mucosa and direct neurogenic
mechanisms could be involved in the mechanics of toxicity.
Thus, more than one reaction may occur in a system at
a given time.