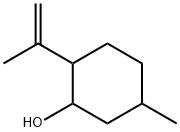
Chemical Properties
Similarly to menthol, isopulegol has three asymmetric carbon atoms and, therefore, four stereoisomers, each
occurring as a pair of enantiomers. All isomers are colorless liquids
with a more or less minty, herbaceous odor.
The isopulegols occur in a number of essential oils, often in optically active
or partly racemic form. Since citronellal readily cyclizes to isopulegol, the latter
occurs frequently in citronellal-containing essential oils, in which it is formed during
the isolation of the oil.
(1R,3R,4S)-(?)-Isopulegol is produced on a large scale as an intermediate for the
production of (?)-menthol.(br/)
A huge number of catalysts for the cyclization of citronellal to isopulegol have
been described. Newer developments offer advantages in diastereoselectivity and
yield through the use of modified silicon dioxides or zeolites.
Isopulegol is mainly used in mint-like flavor compositions. Pure (?)-isopulegol
is also used to impart cooling sensation effects to cosmetic and pharmaceutical
preparations.
Terpineols are unsaturated monocyclic terpene alcohols and are formed by
acid-catalyzed hydration of pinenes;α-, β-, γ-, and δ-isomers exist:
α- and β-Terpineol occur in optically active forms and as racemates. α-Terpineol
is an important commercial product. It occurs in a large number of essential oils
primarily as (?)-α-terpineol (e.g., in conifer and lavandin oils). Small quantities
of (+)- and rac-α-terpineol are found in many other essential oils; β-, γ-, and δ-terpineol do not occur widely in nature.