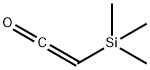
Physical properties
bp 81–82 °C; d 0.80 g cm?3.
Uses
Trimethylsilylketene is a reactive acylating agent for amines and alcohols; building block
for synthesis of coumarins; synthesis of α-silyl ketones via
the addition of organocerium reagents; treatment with stabilized
ylides forms trimethylsilyl-substituted allenes;a cycloaddition
with aldehydes affords β-lactones; forms small rings with
diazomethane; treatment with n-BuLi forms a ketene enolate. It participates in the reactions of Trimethylsilylacetylation of Alcohols and Amines, Synthesis of Coumarins via Cyclization–Elimination, One-pot Formation of α-Silyl Ketones, Preparation of Trimethylsilyl-Substituted Allenes, Preparation of β-Lactones, Reaction with Diazomethane to Form Silylated Cyclopropanes
and Cyclobutanones, Synthesis of Heterocycles, Formation of the Ketene Enolate, and other uses.
Preparation
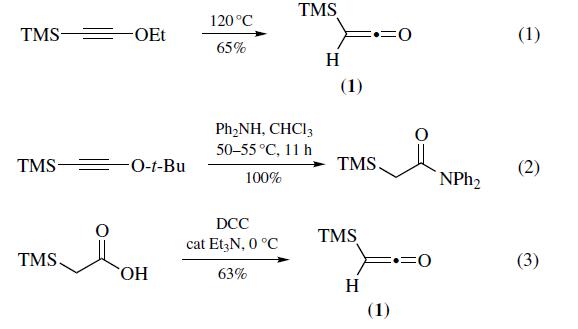
Most often prepared (eq 1) by pyrolysis
of ethoxy(trimethylsilyl)acetylene at 120??C (100 mmol scale,
65% yield).Recently, pyrolysis of t-butoxy(trimethylsilyl)
acetylene has been shown to be a convenient alternative for
the preparation of trimethylsilylketene (1). Thermal decomposition
of t-butoxy(trimethylsilyl)acetylene causes elimination
of 2-methylpropene slowly at temperatures as low as 50??C and
instantaneously at 100¨C110??C (30 mmol scale, 63% yield).
The main advantage of this method is that it is possible to generate
trimethylsilylketene in the presence of nucleophiles, leading
to in situ trimethylsilylacetylation (eq 2). Increased shielding
of the triple bond prevents problems such as polymerization
and nucleophilic attack that occur when the ketene is generated
in situ from (trimethylsilyl)ethoxyacetylene. Trimethylsilylketene
can also be prepared (eq 3) via the dehydration
of commercially available trimethylsilylacetic acid with 1,3-
dicyclohexylcarbodiimide (DCC) in the presence of a catalytic
amount of triethylamine (100 mmol scale, 63%). Other
typical methods used for ketene generation such as dehydrohalogenation
of the acyl chloride and pyrolysis of the
anhydride have been applied to the preparation of (1);
however, both methods afford low yields.
There have been no significant developments in the methods
used to prepare trimethylsilylketene (TMSK). However,
Black et al. have published slight modifications. to the original
preparation by Ruden, which primarily deals with accessing
ethoxyacetylene.
storage
Trimethylsilylketene is unusually stable for an aldoketene with respect to dimerization and decomposition. Samples stored neat under nitrogen at room temperature show no noticeable decomposition after several months.
Purification Methods
Purified by distillation at 82 °C/760 mmHg.