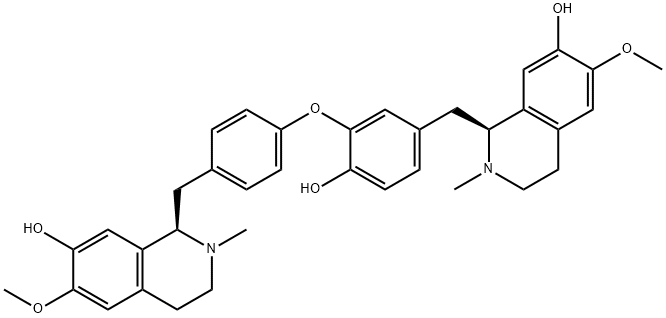
MAGNOLINE
- Product NameMAGNOLINE
- CAS6859-66-1
- CBNumberCB1480557
- MFC36H40N2O6
- MW596.71
- MDL NumberMFCD07781417
- MOL File6859-66-1.mol
- MSDS FileSDS
Chemical Properties
| Melting point | 178-179° |
| alpha | D -9.6° (pyridine) |
| Boiling point | 738.3±60.0 °C(Predicted) |
| Density | 1.254±0.06 g/cm3(Predicted) |
| pka | 9.31±0.45(Predicted) |
| FDA UNII | 850A38GCSI |