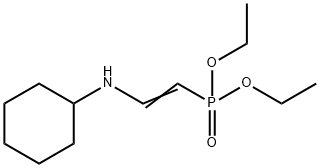
Preparation
Preparative Methods of Diethyl β-(Cyclohexylimino)ethylphosphonate: (EtO)2CHCH2P(O)(OEt)2 is prepared by reaction of Triethyl Phosphite with
BrCH2CH(OEt)2. Acetal hydrolysis affords diethyl formylmethylphosphonate, which is combined with an
equimolar quantity of cyclohexylamine in MeOH. The product is isolated by fractional distillation in the
presence of K2CO3, to prevent acid-catalyzed dimerization, and at reduced pressure, followed by
crystallization from cold pentane.
[1b] Yields are 65-75%. A modified procedure for imine formation uses
MeCN as solvent with isolation of product from Et2O at -40 °C to afford white crystalline material in 70%
yield.
[2]
References
1. (a) Nagata, W.; Hayase, Y. TL 1968, 4359. (b) Nagata, W.; Wakabayashi, T.; Hayase, Y. OS 1973, 53, 44. (c)
Nagata, W.; Wakabayashi, T.; Hayase, Y. OS 1973, 53, 104. (d) Nagata, W.; Hayase, Y. JCS(C) 1969, 460.
2. Farnum, D. G.; Ghandi, M.; Raghu, S.; Reitz, T. JOC 1982, 47, 2598.