Uses
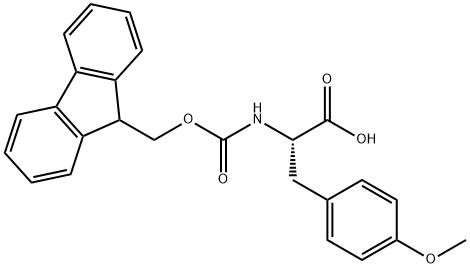
Fmoc-p-methoxy-L-phenylalanine is a phenylalanine derivative protected by the Fmoc (9-fluorenylmethoxycarbonyl) group. The Fmoc protecting group is easily removed under mild conditions without destroying the amino acid's activity, making this compound an indispensable raw material in peptide synthesis. It can also be used as a key intermediate in the synthesis of ACE inhibitors for treating hypertension.
Description
Fmoc-Tyr(Me)-OH, also known as Fmoc-O-methyl-L-tyrosine, is an Fmoc protected tyrosine derivative that is potentially useful for proteomics studies and solid phase peptide synthesis techniques. Tyrosine is a important amino acid - one of the few amino acids which is phosphorylated to vary the physical properties of the peptides, and is a precursor for the formation of iodinated thyroid hormones. The Fmoc group is typically removed with a base such as pyridine - an orthogonal de-protection strategy to the acid labilie Boc group.
Chemical Properties
White to off-white powder
reaction suitability
reaction type: Fmoc solid-phase peptide synthesis
Synthesis
Fmoc-Tyr(Me)-OH, also known as Fmoc-Tyr(OMe)-OH, was obtained from Fmoc-Tyr(OMe)-OMe within 1h and was isolated following protocol 1 or 2 in 96% yield[1]. Under argon atmosphere, anhydrous THF (2.0 mL) was added to the protected amino acid Fmoc-Tyr(OMe)-OMe (0.12 mmol) and to MgI2 * (1.2 mmol). The suspension was heated in a sealed reactor using microwave irradiation at 120°C. A Na2S2O3 aqueous solution (0.1 M) was then added and the resulting homogeneous mixture was directly analysed by analytical HPLC and LC/MS. To isolate the product, two protocols could be applied:
Protocol 1: The crude material was purified by preparative HPLC.
Protocol 2: The crude material was extracted with AcOEt (x3). The organic layer was washed with brine, dried over MgSO4, filtered and concentrated under reduced pressure. The residue was diluted in H2O/EtOH and lyophilized.
References
[1] Berthet M, et al. MgI2 -Mediated Chemoselective Cleavage of Protecting Groups: An Alternative to Conventional Deprotection Methodologies. Chemistry - A European Journal, 2015; 21: 11014-11016.