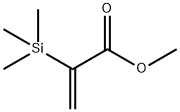
Physical properties
bp 75 °C/50 mmHg, 152 °C/690 mmHg.
Uses
Methyl 2-Trimethylsilylacrylate is widely used as reactive, α-silyl Michael acceptor for conjugate addition of carbanions,
enolate anions, organolithium reagents, and Grignard
reagents; affords 3-cyclopentenecarboxylates and cyclohexanecarboxylates
by [3 + 2] and [4 + 2] annulation methods; provides
α-silyl esters and vinylsilanes by organolithium conjugate
additions.
Preparation
Methyl 2-Trimethylsilylacrylate is prepared by the reaction of (E)-2-
(trimethylsilyl)vinyllithium with methyl chloroformate or by
carbonation of the corresponding Grignard reagent prepared
from (1-bromovinyl)trimethylsilane, giving 2-trimethylsilylacrylic
acid. The methyl ester is prepared from the acid
by direct esterification with absolute methanol in the presence
of mineral acid, by reaction with diazomethane at low temperature,
or by treatment with BF3·MeOH complex.