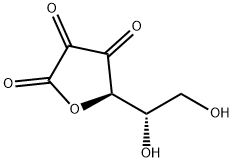
DEHYDROASCORBIC ACID
- Product NameDEHYDROASCORBIC ACID
- CAS490-83-5
- CBNumberCB1397000
- MFC6H6O6
- MW174.11
- EINECS207-720-6
- MDL NumberMFCD00066467
- MOL File490-83-5.mol
- MSDS FileSDS
Chemical Properties
| Melting point | 228 °C (dec.)(lit.) |
| alpha | D20 +56° |
| Boiling point | 389.3±38.0 °C(Predicted) |
| Density | 1.743±0.06 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | DMSO (Slightly, Heated), Methanol (Slightly, Sonicated) |
| form | Solid |
| pka | 3.90(at 25℃) |
| color | White to Dark Orange |
| Merck | 13,2886 |
| Stability | Light Sensitive |
| InChI | 1S/C6H6O6/c7-1-2(8)5-3(9)4(10)6(11)12-5/h2,5,7-8H,1H2/t2-,5+/m0/s1 |
| InChIKey | SBJKKFFYIZUCET-JLAZNSOCSA-N |
| SMILES | OC[C@H](O)[C@H]1OC(=O)C(=O)C1=O |
| LogP | -1.702 (est) |
| CAS DataBase Reference | 490-83-5(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | Y2Z3ZTP9UM |
Safety
| Symbol(GHS) |

|
|||||||||
| Signal word | Warning | |||||||||
| Hazard statements | H302 | |||||||||
| Precautionary statements | P264-P270-P301+P312+P330-P501 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 22 | |||||||||
| WGK Germany | 3 | |||||||||
| F | 1-8-10 | |||||||||
| HS Code | 2936270000 | |||||||||
| Storage Class | 11 - Combustible Solids | |||||||||
| NFPA 704: |
|



