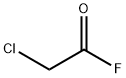
Preparation
Chloroacetyl Fluoride is generally prepared by fluorination of ClCH2COCl. A variety of
reagents has been employed for this transformation, including benzoyl fluoride/KH2F, KHF2, KF, KF/tetraalkylammonium chloride, and dialkylaminosulfur trifluoride.
Preparation of ClCH2COF may also be accomplished from ClCH2CO2H through the use of 2-
chloro-1,1,2-trifluoroethyldiethylamine as the fluoride source. Treatment of
ClCH2CF2CF2OCH3 with SO3 leads to the formation of ClCH2COF in 94% yield, which is the
highest yield reported for the preparation of the reagent.
storage
Chloroacetyl Fluoride is corrosive, moisture sensitive, and should be stored
under anhydrous conditions. This reagent should be handled in a fume hood.