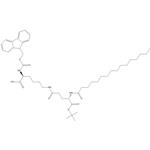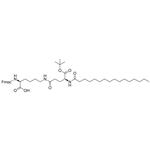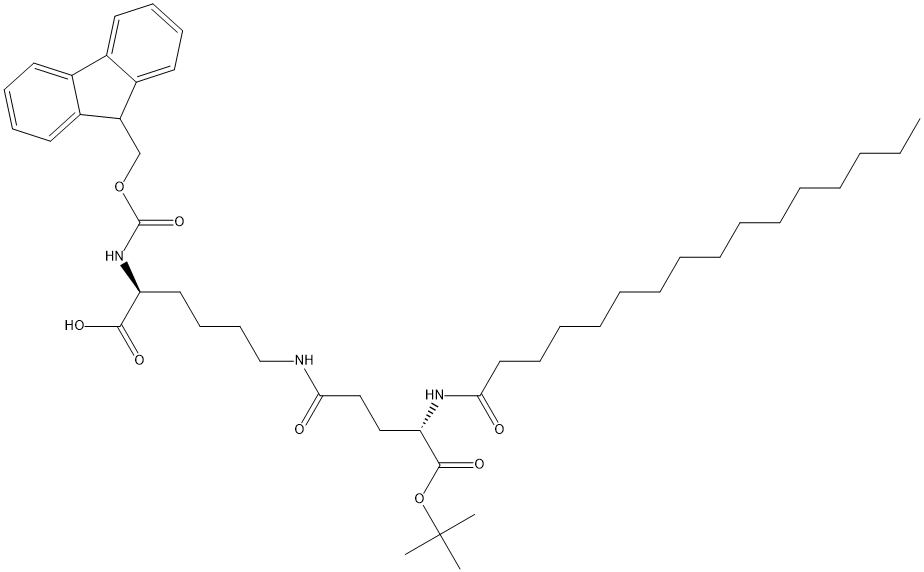
Fmoc-Lys(Pal-Glu-OtBu)-OH
- Product NameFmoc-Lys(Pal-Glu-OtBu)-OH
- CAS1491158-62-3
- CBNumberCB13054762
- MFC46H69N3O8
- MW792.06
- MDL NumberMFCD27952849
- MOL File1491158-62-3.mol
Chemical Properties
| Boiling point | 944.2±65.0 °C(Predicted) |
| Density | 1.099±0.06 g/cm3(Predicted) |
| pka | 3.88±0.21(Predicted) |
| form | Solid |
| color | White to off-white |
| InChIKey | SUFBOZUGILQOQW-FMQAFZDONA-N |
| SMILES | C(O)(=O)[C@@H](N(C1C2=C(C=CC=C2)C2=C1C=CC=C2)C(OC)=O)CCCCNC(=O)CC[C@H](NC(=O)CCCCCCCCCCCCCCC)C(OC(C)(C)C)=O |&1:3,31,r| |
Fmoc-Lys(Pal-Glu-OtBu)-OH Price
| Product number | Packaging | Price | Product description | Buy |
|---|---|---|---|---|
| TRC H950213 | 50mg | $155 | (2S)-2-(9H-fluoren-9-ylmethoxycarbonylamino)-6-[[(4S)-4-(hexadecanoylamino)-5-[(2-methylpropan-2-yl)oxy]-5-oxopentanoyl]amino]hexanoicacid |
Buy |
| ChemScene CS-0038825 | 1g | $230 | Fmoc-Lys(Pal-Glu-OtBu)-OH |
Buy |
| ChemPep 181247 | 1g | $250 | Fmoc-Lys(Pal-Glu-OtBu)-OH |
Buy |
| ChemScene CS-0038825 | 100mg | $60 | Fmoc-Lys(Pal-Glu-OtBu)-OH |
Buy |
| ChemScene CS-0038825 | 250mg | $115 | Fmoc-Lys(Pal-Glu-OtBu)-OH |
Buy |
Fmoc-Lys(Pal-Glu-OtBu)-OH Chemical Properties,Usage,Production
Uses
Fmoc-Lys(Pal-Glu-OtBu)-OH can be used as organic synthesis intermediate and pharmaceutical intermediate, mainly used in laboratory research and development process and chemical production process.Uses
Fmoc-Lys (Pal-Glu-OtBu)-OH is a non-cleavable antibody-drug conjugates linker capable of synthesizing antibody-drug conjugates. It can also be used as an alkyl chain-based PROTAC linker for the synthesis of PROTACs.Application
Fmoc-Lys(Pal-Glu-OtBu)-OH is a racemic, solid-phase, industrial building block for the synthesis of peptides and polypeptides. This product is used in the synthesis of liraglutide (a peptide), which is used to treat type 2 diabetes mellitus. It is synthesized by a solid phase process using coupling reactions with an acid labile linker. This product has been shown to be an efficient building block for the synthesis of peptides and polypeptides that have a sequence that can be varied by changing the protecting group on the amino acid.Biological Activity
ADCs are comprised of an antibody to which is attached an ADC cytotoxin through an ADC linker[1]. PROTACs contain two different ligands connected by a linker; one is a ligand for an E3 ubiquitin ligase and the other is for the target protein. PROTACs exploit the intracellular ubiquitin-proteasome system to selectively degrade target proteins[2].Synthesis
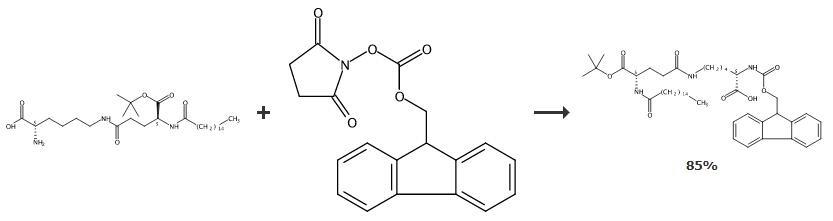
In a flask aqueous sodium carbonate (1.8 g) was prepared followed by addition of THF (800 mL) and then this mixture was cooled to 0-10 0C. To this mixture, (S)-2-((((9Hfluoren-9-yl)methoxy)carbonyl)amino)-6-aminohexanoic acid (4.59 g) was added. Then, a solution of 1 -(tert-butyl) 5-(2,5-dioxopyrrolidin-1-yl) palmitoyl-L-glutamate (corresponding to 5 g of 5-(tert-butoxy)-5-oxo-4-palmitamidopentanoic acid) prepared according to example 2, was slowly added to above mixture at about 5 0C. The reaction mixture was stirred for about 2 hours at 25 0C followed by addition of water (600 mL). The solvents were subjected to distillation and then the pH of the obtained compound is adjusted to 3.0 using 5N hydrochloric acid. The mixture was stirred for 1-2 hours and resulting solid was isolated filtration, washing with water (30 mL). The obtained solid was dried under vacuum for 8 hours at about 45°C to afford title compound in -85% yield.
References
[1] Beck A, et al. Strategies and challenges for the next generation of antibody-drug conjugates. Nat Rev Drug Discov. 2017;16(5):315-337.[2] Nalawansha DA, et al. PROTACs: An Emerging Therapeutic Modality in Precision Medicine. Cell Chem Biol. 2020;27(8):998-985.
Preparation Products And Raw materials
Fmoc-Lys(Pal-Glu-OtBu)-OH Supplier
Global(126)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | ||
|---|---|---|---|---|---|---|
| +8617327281506 | market@chemtour.com | China | 1519 | 58 | ||
| 16314854226; +16314854226 |
inquiry@bocsci.com | United States | 19654 | 58 | ||
| +86-0592-7761068 +8617350879715 |
changaiwei@sinopeg.com | China | 176 | 58 | ||
| +86-028-64841719 +8617390183901 |
daisy@enlaibio.com | China | 1106 | 58 | ||
| +86-15542445688 | sales@alphabiopharm.com | China | 992 | 58 | ||
| +86-(0)57185586718 +86-13336195806 |
sales@capot.com | China | 29730 | 60 | ||
| +undefined-21-51877795 | ivan@atkchemical.com | China | 33024 | 60 | ||
| +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29863 | 58 | ||
| 028-82550498 | export@pu-kang.com | CHINA | 210 | 58 | ||
| 18853181302 | sale@chuangyingchem.com | CHINA | 5906 | 58 |
View Lastest Price from Fmoc-Lys(Pal-Glu-OtBu)-OH manufacturers
1491158-62-3, Fmoc-Lys(Pal-Glu-OtBu)-OHRelated Search
- FMOC-D-4-Chlorophe
- FMOC-LYS(BOC)(ISOPROPYL)-OH
- Fmoc-Lys(nicotinoyl)-OH
- (R)-N-Acetyl-2-naphthylalanine
- Fmoc-D-Asn(Trt)-OH
- 4-[(AMinocarbonyl)aMino]-N-[(9H-fluoren-9-ylMethoxy)carbonyl]-D-phenylalanine
- Fmoc-D-homoArg(Et)2-OH·HCl
- (R)-N-Fmoc-(3-Pyridyl)alanine
- N-[(9H-Fluoren-9-ylMethoxy)carbonyl]-4-[[[(4S)-hexahydro-2,6-dioxo-4-pyriMidinyl]carbonyl]aMino]-L-phenylalanine
- Fmoc-L-beta-Homoarginine(Pmc)
1of4
PROMPT×
PROMPT
The What'sApp is temporarily not supported in mainland China
The What'sApp is temporarily not supported in mainland China
Cancel
Determine